Sonoluminescence — the emission of light from bubbles in a liquid that has been excited by sound — is a mystery. How does a sound wave put enough energy into such a small volume as to cause light to be emitted? The concentration of energy needed is something like a factor of one trillion, according to this Los Alamos National Laboratory introduction to the phenomenon. And not only that; the spectrum of the emitted light implies extremely high temperatures. Fusion, anyone?
 Well, not yet. But the slang term for sonoluminescence, ‘star in a jar,’ seems a little closer to reality now that the first direct measurements of the phenomenon are in. They show that the temperature inside a collapsing bubble can reach 20,000 degrees Kelvin, which is four times the temperature of the surface of the Sun. This work, by Ken Suslick and David Flannigan (University of Illinois at Urbana-Champaign), comes two years after controversial findings by an Oak Ridge National Laboratory team that found evidence of fusion in sonoluminescence experiments, but the Illinois researchers are more circumspect. They claim only that plasma can be formed in the process, and it is known that confined fusion reactions require a plasma.
Well, not yet. But the slang term for sonoluminescence, ‘star in a jar,’ seems a little closer to reality now that the first direct measurements of the phenomenon are in. They show that the temperature inside a collapsing bubble can reach 20,000 degrees Kelvin, which is four times the temperature of the surface of the Sun. This work, by Ken Suslick and David Flannigan (University of Illinois at Urbana-Champaign), comes two years after controversial findings by an Oak Ridge National Laboratory team that found evidence of fusion in sonoluminescence experiments, but the Illinois researchers are more circumspect. They claim only that plasma can be formed in the process, and it is known that confined fusion reactions require a plasma.
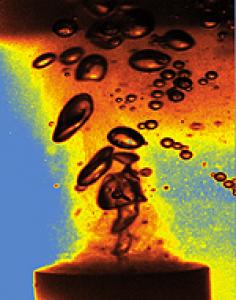
Image: A cloud of gas bubbles in a liquid excited by ultrasound (generated by a titanium rod vibrating 20,000 times a second) can emit flashes of light (sonoluminescence) due to extreme temperatures inside the bubbles as they collapse. A single bubble can be trapped in an ultrasonic field and driven into oscillation will flash on every cycle when it reaches maximum compression. Photo by K.S. Suslick.
The explosive collapse of bubbles of gas in a liquid is known as cavitation. An article posted online at Nature.com explains sonoluminescence as performed by Suslick and Flannigan:
The sound waves (between 20 and 40 kilohertz) produce areas of high and low density within the liquid, making pressure at any one point oscillate between two extremes. Bubbles of gas in the liquid swell rapidly at lower pressures before being squeezed tight by the high pressure that follows.
The change in pressure is so fast that the bubble effectively implodes with enough force to generate tremendous heat, in a process called acoustic cavitation. “Compress a gas and you heat it, just like pumping up a bicycle tire,” explains Suslick. The heat separates electrons from their atoms, and as they snap back into position the energy they acquired is released as a burst of light.
The temperatures thus far recorded are remarkable, as noted in this UIUC press release:
“At 20,000 degrees Kelvin, this emission originates from the plasma formed by collisions of atoms and molecules with high-energy particles,” Suslick said. “And, just as you can’t see inside a star, we’re only seeing emission from the surface of the optically opaque plasma.” Plasmas are the ionized gases formed only at truly high energies.
Suslick and Flannigan worked with sulfuric acid containing traces of argon gas instead of the water used in previous experiments. The resultant release of light was 2700 times more powerful, and clearly points to continued investigation of different gas/liquid mixtures. Suslick offers a Web page on sonoluminescence at UIUC.
‘Bubble fusion’ has yet to be proven, but clearly sonoluminescence is becoming more and more promising in studying how highly energized plasma forms. Suslick and Flannigan’s paper “Plasma formation and temperature measurement during single-bubble cavitation” appears in the March 3 issue of Nature (Vol. 434, pp. 52 – 55); an abstract can be read here. The Oak Ridge team’s paper is Taleyarkhan et al., “Evidence for Nuclear Emissions During Acoustic Cavitation,” Science Vol. 295, pp. 1868-1873 (2002).

