Years ago I wrote a story called ‘Rembrandt’s Eye,’ using as background a planet whose foliage was predominantly red. The story, which ran in a short-lived semi-pro magazine called Just Pulp, came back to mind when the news from Caltech arrived. Researchers at the Virtual Planetary Laboratory there now believe that Earth-type worlds may have foliage that is largely yellow, orange or, as in the case of my planet, red. The green of Earth’s plant life is anything but a universal standard.
This interesting conclusion emerges from computer models designed to provide pointers for the future search for plant life on exoplanets. After all, astronomers will need to know what they might see in the spectra we’ll one day be able to harvest from space-borne observatories. Ponder everything that’s involved, from the color of the main sequence primary star to the aquatic habitats of aqueous plants. The search involves the way photosynthesis might occur under varying conditions, with the filtering effect of planetary atmospheres as a major player.
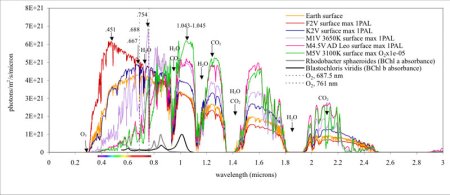
Image (click to enlarge): This graph shows the intensity of light by color (wavelength) that reaches the surface of Earth-like planets orbiting different types of stars. From hotter to cooler, the star types are F, G, K, and M. Our Sun is a G2 star (yellow line). A planet orbiting an F2 star (red line) has more blue light at the surface, whereas Earth and the K2 star planet receive more red light. Planets around M stars receive much less visible light but much more infrared light. Atmospheric gases such as ozone (O3), oxygen (O2), water vapor (H2O), and carbon dioxide (CO2) absorb light at specific wavelengths, producing the pronounced dips that astronomers might someday detect. Then in the diagram’s horizontal axis, mark the wavelengths from 0 to 0.4 microns as UV, 0.4 to 0.7 as visible, and longer than 0.7 as infrared. Credit: NASA.
Also affecting foliage color are factors like stellar flare activity, the chemical reactions stellar radiation causes in the atmosphere, the role of ozone, carbon dioxide and water vapor, the amount of water available and the quantity of light that reaches the surface. As the simulations ran, a wide variety of habitable scenarios came into play, including one that removed most of the ozone that shields against surface radiation.
Surprisingly, survivable habitats may occur even in places like this in a ‘sweet spot’ below the surface of the water, says Victoria Meadows (Caltech VPL):
“We found that the sweet spot could be up to nine meters underwater for a planet orbiting a star significantly cooler than our sun, and photosynthesis could still take place. Something with a floatation capability could be protected from solar flares and still get enough photons to carry on.”
On Earth, plants absorb blue and red light while reflecting away large amounts of green. But the dominant color on other planets depends on so many different factors in the atmosphere and the light emitted by the planet’s star that not even infrared can be ruled out for photosynthesis. Some of the more exotic landscapes of science fiction authors may yet be realized, though doubtless in ways that will continue to surprise us.
The papers are Kiang et al., “Spectral signatures of photosynthesis I: Review of Earth organisms” (abstract here) and “Spectral signatures of photosynthesis II: coevolution with other stars and the atmosphere on extrasolar worlds” (abstract), both slated to appear in a forthcoming issue of Astrobiology. Also see this NASA backgrounder.


Is it really certain, though, that the color of plants on a given planet is a function of optimal light absorption? I mean, Sol’s peak wavelength is slightly greenward of yellow, and yet Earth plants reflect green light!
It was proposed in an article I read once in DISCOVER Magazine (not an ideal source, I know) that the first photosynthetic organisms to evolve were red-purple algae that absorbed the yellow and green light. So the things living underneath them in ponds and such weren’t getting any yellow or green light, just red and blue, so they had to evolve a pigment that absorbed those colors preferentially, and that was chlorophyll (which absorbs red and blue and reflects yellow and green). And for whatever reason, maybe just the luck of the draw, it was the also-rans that ended up becoming dominant later on. If evolution had gone a bit differently, this theory says, plant life on Earth could have easily been purple instead of green.
Christopher, re light absorption I’m no expert, but let me quote from this Caltech press release that may be useful:
“The researchers focused on the way plants use light for energy to produce sugar–which is pretty much the definition of photosynthesis–because photosynthetic pigments must be adapted to the available light spectrum. The available light spectrum at a planet’s surface is a result of both the light from the parent star and filtering effects of gases in the atmosphere. For example, ozone absorbs ultraviolet light, so that not much reaches Earth’s surface.
“It turns out that the spectrum of the number of particles of light is what is important, and on Earth there are more particles in the red,” Kiang explains. “This could explain why plants here on Earth are mainly green.”
But opinions from others on these matters would be helpful.
The above quotes Nancy Kiang, a biometeorologist at NASA’s Goddard Institute for Space Studies, who is currently working at Caltech.
I’m going on memory only here, but I think photosynthesis on Earth is in the 2% efficiency range for conversion of sunlight to energy (Sorry, no green-skinned human mutations – not enough square footage of exposed skin!) But I wonder if there’s a theoretical upper limit to how efficient photosynthesis could be on other worlds? Is it possible that any plant life under some of the conditions described could actually be much more efficient that ours?
I read a bit more about this on New Scientist’s site, and it says: “Terrestrial photosynthesis depends mostly on red light, the most abundant wavelength reaching the Earth’s surface, and blue light, the most energetic.”
http://space.newscientist.com/article/dn11578-for-plants-on-alien-worlds-it-isnt-easy-being-green.html
So that’s offering a different theory from the one I mentioned above. I can see the part about blue light being the most energetic, but is red really more abundant? And if so, why?
http://www.physorg.com/news95605211.html
I just mentioned this article for another thread here, but the article pertains to this thread too.
Edg
Bear in mind that for many plants overheating of leaves is a serious problem. This may be the reason why plants are not simply black – there is a counterpressure to reflect some of the energy away.
Well the quantum effect article cited explains how plants do it without heating up the leaves, the quantum computation of the most efficient path.
As for the overheating issue, that would be less of a factor under a dim red star; indeed, plants would probably need to absorb as much energy as they could. So they might be very dark, even black, on such planets.
A piece of parochialism that almost no one notices, when speaking of energy or number of photons required to fix one molecule of CO2, is the assumption that the net process is going to be CO2 + H2O -> C(H2O) + O2
This is the net reaction for most photosynthesis on Earth. But in an atmosphere dominated by CO, the reaction would be 2CO + H2O -> C(H2O) + CO2, which is so cheap that it requires almost no external source of energy, and could easily be carried forward with infrared photons. If the atmosphere were dominated by methane or ethane (as in your prototype carbon planet), photosynthetic organisms would have to find a way to get rid of excess hydrogen: if no “electron acceptor” is available, generate hydrogen gas.
It is a pity there is still no link to a proper peer reviewed version of this paper because there are so many glaring calculation errors in this draft paper which undermines many of their conclusions
For example.
In the second paper, “Spectral signatures of photosynthesis II: coevolution with other stars and the atmosphere on extrasolar worlds”
Many of the figures given in “Table 2a. Maximum surface and underwater incident photon flux densities for cloudless planets at solar noon” for a G2V Earth/sol would make the figures in “Table 2b. Average surface and underwater incident photon flux densities for cloudless planets, illuminated face” for a G2V Earth/sol planet are simply impossible.
Because if, as the table 2b says, Earth receives 0.18 x 10 (to the power of 20) photons in the UV-B 280-315 nm range per square meter per second at noon then, it is quite obviously impossible that the Earth could ever receive more than 0.09 x 10 (to the power of 20) photons per square meter per second on average, for the cloudless sunlit face. As, sunlight is shining on twice as much surface area and therefore with at least half the intensity, on the sunlit side of the Earth on average; than it does on the part of the Earth it shines directly above on, at noon.
Yet, table 2b claims that a G2V Earth would receive 0.17 x 10 (to the power of 20) photons in the UV-B 280-315 nm range per square meter per second, on average on the cloudless sunlit side. This is considerable more than half the amount it receives at noon!
The paper use the figures in Table 2a and 2b to make dubious claims about ultraviolet light levels being much lower for Earth like planets orbiting K2V and F2V type stars than for Earths orbiting G2V stars. Correcting the figures in 2b, assuming Table 2a was accurate, could actually make conditions for the K2V scenario much more like a G2V, with almost identical levels of ultraviolet light, but would show how conditions in the F2V scenario were much less comfortable and possibly even hostile to G2V Earth like life.