A new look at Stanley Miller’s experiments at the University of Chicago in the early 1950s offers up an intriguing speculation: Volcanic eruptions on the early Earth may have been crucial for the development of life. Miller used hydrogen, methane and ammonia to re-create what was then believed to be the the primordial atmosphere on our planet, operating with closed flasks containing water in addition to the gases. An electric spark was then used to simulate lightning, and as anyone who has ever cracked a textbook knows, the water became laden with amino acids after a few weeks.
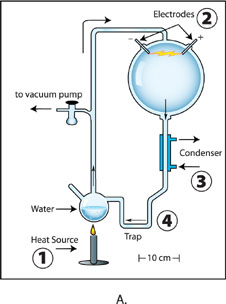
Image A: The apparatus used for Miller’s original experiment. Boiled water (1) creates airflow, driving steam and gases through a spark (2). A cooling condenser (3) turns some steam back into liquid water, which drips down into the trap (4), where chemical products also settle. Credit: Ned Shaw, Indiana University.
It never occurred to me that samples from the original experiments might have survived after all these years, but fortunately Jeffrey Bada (University of California at San Diego) discovered them after Miller’s death in 2007. And given the increasing sophistication of our tools for chemical analysis, it was a natural move to look for chemicals within those samples that might have eluded detection fifty years ago. Working with and re-interpreting old data is fascinating enough and is going to become more and more common in all the sciences, now that computers have given us the ability to generate and store such vast quantities of information. But re-analyzing samples from experiments as historic as these to pull out new insights puts a bit of a chill down the spine. If only Miller could have known about this work!
Miller’s three different experiments included one that injected steam into the gas to simulate a volcanic cloud, and it turns out that it is that experiment that produced the widest variety of compounds. The work, performed at NASA GSFC, turned up fruitful results indeed, some 22 amino acids, ten of which were new to this kind of experiment. A key factor is the change in our thinking about the ancient atmosphere, which is now believed to have been composed mostly of carbon dioxide, carbon monoxide and nitrogen rather than the mix Miller originally used.
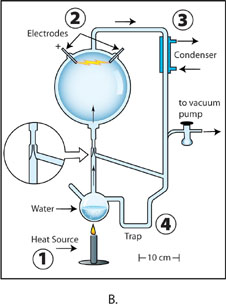
Image B: The apparatus used for Miller’s “second,” initially unpublished experiment. Boiled water (1) creates airflow, driving steam and gases through a spark (2). A tapering of the glass apparatus (inlay) creates a spigot effect, increasing air flow. A cooling condenser (3) turns some steam back into liquid water, which drips down into the trap (4), where chemical products also settle. Credit: Ned Shaw, Indiana University.
How do changing views of the atmosphere affect the outcome? Daniel Glavin (GSFC), who analyzed the samples at Goddard, has this to say:
“At first glance, if Earth’s early atmosphere had little of the molecules used in Miller’s classic experiment, it becomes difficult to see how life could begin using a similar process. However, in addition to water and carbon dioxide, volcanic eruptions also release hydrogen and methane gases. Volcanic clouds are also filled with lightning, since collisions between volcanic ash and ice particles generate electric charge. Since the young Earth was still hot from its formation, volcanoes were probably quite common then. The organic precursors for life could have been produced locally in tidal pools around volcanic islands, even if hydrogen, methane, and ammonia were scarce in the global atmosphere. As the tidal pools evaporated, they would concentrate the amino acids and other molecules, making it more likely that right sequence of chemical reactions to start life could occur. In fact, volcanic eruptions could assist the origin of life in another way as well – they produce carbonyl sulfide gas, which helps link amino acids into chains called peptides.”
Jeffrey Bada, a co-author of the paper on this work, was Miller’s graduate student between 1965 and 1968, and continued working with the scientist in the intervening years. In addition to its provocative insight into the potential role of volcanic activity, the new work is a reminder that the things we do sometimes survive us in the most unexpected ways. I’m thinking of a particular researcher I once worked with, now gone, who would have found great pleasure in that notion. The paper is Johnson et al., “The Miller Volcanic Spark Discharge Experiment,” Science 322, No. 5900 (17 October 2008), p. 404 (abstract).


Ah, there’s nothing like the invocation of the Miller-Urey experiment to set the creationist/IDist’s juices flowing. :-)
Hi All
The CO/CO2/N2 mix is by no means a certainty. Several lines of evidence suggest more than a trace of methane and hydrogen. In fact hydrogen loss to space might have been so inhibited that it built up over time to ~40% of the atmosphere.
Interesting chemistry indeed. I do wonder if the discoveries by Miller and others about the enhancement of oligomerisation in ice might mean that Life itself began in icy asteroids and comets, not on Earth.
The more intriguing question in connection with the Miller-Urey experiment is chirality. I believe that the resulting compounds of all such experiments are racemic, which to me at least implies an early selection/discrimination step in the chain of chemical reactions that gave rise to the first cells.
As I understand this (and please correct me if I’m wrong, Athena), amino acids exist in two chiral forms that are essentially mirror images of each other. And the Miller-Urey experiments seem to produce equal numbers of each form (i.e., a racemic result), whereas in nature we find only the L-form. That early selection step you talk about seems striking, but do we know what would cause it? I’m curious about any current work on this.
Hi Folks;
I saw a TV National Geographic special last night on the record setting deep dive of the Trieste Bathyscaph several decades ago. Part of the progam included a presentation of the tube worms, bacteria, clams, and crabs that can be found thriving near hydrothermal volcanic vents at a depth of about 1 1/2 km to 3 km below the ocean surface.
The presense of these thriving ecosystems leads me to have hope that we will find simmilar or analogous organisms below the sub-surface ice of Europa, perhaps in sub-surface aquatic regions below the surface of Enceladus, and/or on other moons within our solar system.
These sub-marine ecosystems on Earth thrive quite vigorously in the absense of photosynthesis and it was suggested that locations near these hydrothermal vents may have also been a source of rich chemistry in which life on Earth may have started or, at the least, may have been one of multiple contributory environments where at least some of Earth’s organisms have developed.
If planetary systems that are comprised of gas giant planets orbiting sun-like stars or smaller dwarf stars at locations of roughly 1 AU to 10 AU from their host stars are common, then perhaps moons simmilar to Europa, Enceladus, Titan and the works are very common occurances. This possibility leads me to ponder whether or not such sub-surface organisms can develop high degrees of intellegence and perhaps in some cases, technological artifacts.
It is interesting to see how well organized ant, bee, and termite colonies can become with all of the specialized workers within. I find it really interesting that certain ant species actually collect vegetation and use it in underground farm-like locations to grow a certain type of fungus that the respective ants feed on. A short leap of the imagination can lead one to consider whether intellegent organisms could develope without photosynthesis.
Thanks;
Jim
You are right, Paul. All the building blocks of life are chiral (“handed”): not only amino acids, but nucleic acids and sugars as well. It’s like the matter/antimatter asymmetry in cosmology, or the breaking of spin symmetry by some subatomic particles in quantum chromodynamics. That’s also one way we know that something is not terrestrial — by the ratio of R to S (or D to L, or + to -) pre-biomolecules in it.
It is not yet known how this bias got established. There was an interesting theory by John Cairns, who postulated that the relatively fragile chains of biomolecules initially gained stability by forming on a silicon template. The template favored certain geometries, thereby imprinting the resulting molecules. Once the biochains became stable and capable of self-propagation, the scaffolding was discarded and disappeared.
Why did this find have to wait until Miller’s death last year?
Larry, I assume the samples turned up as Miller’s effects were being boxed up, or something like that.
November 11, 2008
Harvard Scientist Asks: Was First Life on Earth Formed by Clay?
In the early 1990s, Jack Szostak of Harvard Medical School began investigating the molecular origins of life in order to understand how chemicals combined to form the first living organisms on primitive Earth.
Inspired by Tom Cech and Sidney Altman’s discovery that RNA could catalyze chemical reactions inside cells (which later earned them a Nobel Prize), Szostak began to explore RNA’s ability to catalyze its own reproduction.
Building on earlier work by other scientists, Szostak and colleagues began experimenting with a clay mixture common on early Earth called montmorillonite, which was found to catalyze the chemical reactions needed to make RNA.
So, did life originally spring from clay as some creation myths assert?
Full article here:
http://www.dailygalaxy.com/my_weblog/2008/11/harvard-scienti.html
Did life begin in a pool of acidic gloop?
19 January 2009 by Douglas Fox
Magazine issue 2691.
JETS of sulphurous steam roar out of holes in the ground and an eggy stench hangs in the air. This is Bumpass Hell, a valley of bubbling mud pools in the heart of the Lassen Volcanic National Park in northern California. The valley is ringed with beautiful pine and fir trees climbing up the surrounding slopes, but life seems to have stayed away from the lower reaches. Billions of years ago, though, the opposite might have been true.
I’ve come to Bumpass Hell with David Deamer, a biochemist from the University of California, Santa Cruz, to watch him run an experiment recreating one of the most important episodes in the history of life: when carbon, hydrogen, oxygen, nitrogen and phosphorus came together in the primordial soup to form amino acids, DNA and the rest of life’s building blocks.
If Deamer is right, then the sort of extreme conditions found here were key to that momentous event. It may be an unattractive and rather dangerous place to work, but to Deamer this is one of the most precious places on Earth – the closest thing he can get to the cauldron of chemicals from which life might have emerged over 4 billion years ago.
Full article here:
http://www.newscientist.com/article/mg20126911.400-did-life-begin-in-a-pool-of-acidic-gloop.html
Methane-eating microbes can use iron and manganese oxides to ‘breathe’
http://www.spaceref.com/news/viewpr.html?pid=28688
“Iron and manganese compounds, in addition to sulfate, may play an important role
in converting methane to carbon dioxide and eventually carbonates in the Earth’s
oceans, according to a team of researchers looking at anaerobic sediments. These
same compounds may have been key to methane reduction in the early, oxygenless
days of the planet’s atmosphere.”
Explosive growth of life on Earth fueled by early greening of planet
http://www.spaceref.com/news/viewpr.html?pid=28687
“Earth’s 4.5-billion-year history is filled with several turning points when
temperatures changed dramatically, asteroids bombarded the planet and life forms
came and disappeared. But one of the biggest moments in Earth’s lifetime is the
Cambrian explosion of life, roughly 540 million years ago, when complex, multi-
cellular life burst out all over the planet.”
“Lost” Miller Experiment Gives Pungent Clue to Origin of Life
03.23.11
GREENBELT, Md. — The origin of life may have been smelly, according to a recent, NASA-funded analysis of residue from a variant of classic experiments performed by Dr. Stanley Miller in the 1950s.
“One of the primary differences between this experiment and others Miller performed is the use of hydrogen sulfide (H2S) gas to help simulate the primordial atmosphere,” said Eric Parker of the Georgia Institute of Technology, Atlanta, Ga.
Hydrogen sulfide gas is commonly known as the awful smell released by rotten eggs. Parker is lead author of a paper on this research appearing the week of March 21 in the on-line Early Edition of the Proceedings of the National Academy of Sciences.
“Much to our surprise, the yield of amino acids from Miller’s hydrogen sulfide experiment is a lot richer than that from any other experiment he had ever conducted,” said Professor Jeffrey Bada of the Scripps Institution of Oceanography, University of California at San Diego, who was a graduate student of Miller’s and is the corresponding author on the paper.
Full article here:
http://www.nasa.gov/centers/goddard/news/releases/2011/lost_exp.html