Although Titan is often cited as resembling the early Earth, the differences are striking. Temperature is the most obvious, with an average of 95 Kelvin (-178 degrees Celsius), keeping water at the surface firmly frozen. Our planet was tectonically active in its infancy, roiled not only by widespread volcanism but also asteroid impacts, especially during the period known as the Late Heavy Bombardment some 4.1 to 3.8 billion years ago.
Throw in the fact that the Earth had high concentrations of carbon dioxide — Titan does not — and it’s clear that we can’t make too broad a comparison between the two worlds. What we do have on Titan, however, is an atmosphere that teems with chemical activity, fueled by light from the Sun and the charged particle environment in the moon’s orbit around Saturn. So we do have a chemistry here that is capable of turning simple organics into more complex ones.
Thus the findings from a new study of archival data using the Atacama Large Millimeter/submillimeter Array (ALMA) are instructive. If we can rule out forms of life as we know them, we can’t exclude combinations that could only form in Titan’s frigid incubator. A potential marker of such chemistry is vinyl cyanide (acrylonitrile), which has been identified in the study and points in interesting directions.
Says lead author Maureen Palmer (NASA GSFC): “The presence of vinyl cyanide in an environment with liquid methane suggests the intriguing possibility of chemical processes that are analogous to those important for life on Earth.”
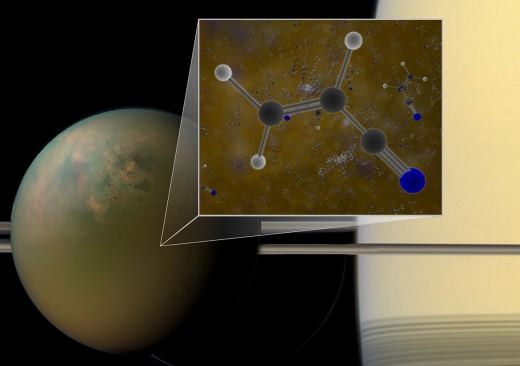
Image: Archival ALMA data have confirmed that molecules of vinyl cyanide reside in the atmosphere of Titan, Saturn’s largest moon. Titan is shown in an optical (atmosphere) infrared (surface) composite from NASA’s Cassini spacecraft. In a liquid methane environment, vinyl cyanide may form membranes. Credit: B. Saxton (NRAO/AUI/NSF); NASA.
An interesting thought, because the three signals Palmer and team found in millimeter wavelength spectra from ALMA observations in 2014 confirm what Cassini had already hinted at, which in turn reinforce laboratory simulations of Titan’s atmosphere. A surface rife with pools of hydrocarbons — and Titan is a place of methane rains, rivers and seas — could allow molecules of vinyl cyanide to link together, forming membranes that resemble the lipid-based cell membranes found on Earth. Titan’s complex organic molecules along with its nitrogen atmosphere and the presence of carbon-based molecules are provocative ingredients.
From the paper:
… lipid membranes, which are common to Earthly organisms, could not exist in cryogenic methane. Cell membrane-like compartments are crucial for the development of life from a sea of prebiotic reactants. These membranes enclose a small volume of solution, where reactants can be concentrated and (pre)biotic reactions can occur with greater frequency than they would in the dilute environment of an entire lake or sea. They also define individual cells as separate from each other, creating the potential for competition and natural selection.
And a nod to recent computational work on this matter:
Recent simulations have investigated some nitrile species for their potential to form flexible membranes in Titan-like conditions. These simulations suggest that vinyl cyanide (C2H3CN; also known as acrylonitrile or propenenitrile) would be the best candidate species for the formation of these hypothesized cell-like membranes, known as “azotosomes”
And now we have vinyl cyanide confirmed on Titan. At Cornell University, Jonathan Lunine worked with Paulette Clancy on a 2015 paper that discussed molecular simulation and its predictions as windows into prebiotic life conditions. Of the ALMA finding, Lunine says this:
“Researchers definitively discovered the molecule, vinyl cyanide (a.k.a. acrylonitrile), that is our best candidate for a ‘protocell’ that might be stable and flexible in liquid methane. This is a step forward in understanding whether Titan’s methane seas might host an exotic form of life. Saturn’s moon, Enceladus is the place to search for life like us, life that depends on — and exists in — liquid water. Titan, on the other hand, is the place to go to seek the outer limits of life — can some exotic type of life begin and evolve in a truly alien environment, that of liquid methane?”
And let’s not forget what else has been demonstrated by this work. What Lunine, Paulette Clancy and James Stevenson modeled in 2015 was exactly the kind of membrane we are now discussing. The finding of vinyl cyanide thus validates the authors’ 2015 paper, pointing to the value of molecular simulations as an adjunct to experimental and observational data.
The next steps? Further laboratory studies of the reactions involving vinyl cyanide. The authors point out that experimental work on membrane formation in cryogenic methane would help us determine just how viable a pathway this might be for astrobiology. We can also use infrared observations of Titan to study the transport of vinyl cyanide to the surface and map its spatial distribution, especially over the moon’s northern seas and lakes.
The paper is Palmer et al., “ALMA Detection and Astrobiological Potential of Vinyl Cyanide on Titan,” Science Advances Vol. 3, No. 7 (28 July 2017) (full text). The 2015 paper on modeling prebiotic environments is Stevenson, Lunine & Clancy, “Membrane alternatives in worlds without oxygen: Creation of an azotosome,” Science Advances Vol. 1, No. 1 (27 February 2015) (full text).



I’ve got my money placed on Titan; this is where we will find something resembling life.
Titan is a chemical factory, this is a good study but remote sensor can only give a peephole of organic chemistry there.
Alternative life system is possible yes, even if it is not found but only is started we could learn a lot from the possibilities there!
Yes this moon is different, less available energy, a few important aminoacids cannot be made at all, but the chemical process there could give insights on how life started on Earth also.
We don’t need to go to another star to have a very interesting world to explore, with submarine, paddleboat, rover and zeppelin.
Sadly such a complex space mission will not be possible in the near future or a lifetime.
I’d like to see what sort of hypothetical metabolism might be possible on Titan. If vesicle compartments can be formed, what sort of compounds do they need to contain and concentrate to create some simple metabolic pathway to extract energy from the environment? In a “metabolism first” abiogenesis, information storage isn’t necessary and we might expect naturally budding vesicles as they “grow”.
Those vesicles would most likely be found in the hydrocarbon lakes and streams, so it is a pity we don’t have a Titan boat or submarine explorer that could look for such “life” as one of its goals.
Good point, Alex. We might be stuck on defining life as using DNA or an analog for information transfer. Maybe there is life wherein information is not as important as metabolism.
Titanian life will be very strange if found, though clearly Huygens landed in a desert or something, since visible azotosome life wasn’t evident. Earth’s largest lifeforms were microbes for ~2 billion years or so, thus we can’t conclude much from that little patch of cryogenic stony ground we’ve already seen.
Why was that area of Titan chosen for Huygens? Or did they have no real say in where the probe landed on that moon?
I am suddenly reminded of Mariner 4 imaging the most “boring” regions of Mars and the Galileo atmosphere probe hitting an atypical “dry” area of the Jovian cloud system.
Often, probe landing sites are chosen for their safety to the probe.
We had no idea at all what the probe was going to touch/splash-down on or in… we didn’t know if there was solid or liquid surfaces and their ratio or extent prior to Cassini’s mapping (groundbased imagery and Hubble during the 90’s helped advance Voyager’s finding or lack thereof) so the probe was outfitted with a penetrometer for hard surface measurements but was also designed to float. It was designed to ‘land’ in a best-guess way yet the landing area was pre-chosen, even after the initial hiccup delay for release.
To Titanic observers, Earth’s organic surface would seem like an impossible ecosystem of roiling acids and solvents.
Are there any further ways we can model proto-organic membranes? Imagine, a ‘periodic table’ of lifelike processes out there, each on distinct chemical and energetic scales. Exobiology could be an unfathomably understated field.
Very good. From the point of view of a hypothetical Titanian, Earth would be a nightmare of solar radiation and oxidation.
The problem with azotosome idea is that it only covers the making of a the cell membrane. What about inside the cell? What kind of catabolism and anabolism would liquid methane support in a cell? It’s certainly can’t involve sugar. What kind of proteins in the DNA would such a cell have? Water is also a better solvent than methane. The chemical reactions would also have to be much slower at lower temperatures.
Reaction rates at Titan’s temperature must be miniscule. I don’t see what the fuss is about. It’s dead, Jim.
That is correct, Titan is an icebox.
The chemical reaction we see come from upper atmosphere where the Sun provide energy.
Organic chemistry but not alive is not important?
Lower down there might be samples on how life got started.
If I could decide, I say: All hands let go of what you are doing, and start to build a Titan mission with submarine, boat, rover and zeppelin.
(No I did not say sample return, even I am realistic – haha!)
Many of these chemicals are the precursors of life’s components, life as you have stated on Titan would be very slow at those temperatures.
Reaction rates for reactions that happen at a reasonable rate on Earth are minuscule on Titan. Life on Titan would have to rely on reactions that happen extremely fast on Earth, reactants that are too unstable at Earth temperatures to keep around to do chemistry with.
Such reactants exist. The real question is what life on Titan would EAT; What’s the ultimate energy source? Or rather, penultimate, as the ultimate energy sources would be the same as on Earth: Volcanic heat, solar radiation.
Presumably one such source would be metastable compounds produced by the action of solar radiation on Titan’s atmosphere. Look for the life in places where such compounds get naturally concentrated, the methane pools around the poles.
The thing is, you can’t expect a lot of life on Titan; Even if there is life there, the Titan biosphere has to be starved for energy.
There is an acetylene sink on Titan… whatever acetylene is produced in the atmosphere disappears once it gets to the surface. It has been suggested that something might be ‘eating’ it. I wouldn’t bet on it but it is intrigueing nonetheless.
In agreement with Geoffrey Hillend and Andrew Palfreyman, because of the extremely low temperatures, and lack of liquid water as a solvent, (bio)chemical reaction rates must be very slow, and any evolution as well: as a rule of thumb, chemical reactions slow down by a factor 2-3 for every 10 K lower. This means that comparable chemistry would be at least on the order of a million times slower on Titan than on early Earth. Even any precursor to life wouldn’t even be close to starting.
Very true, but that’s also the point: It’s a snapshot of pre biochemistry, that might perhaps be heading towards soething life like in a very, very slow way. The interest is the same as that directed at prmitive, orgainic rich asteroids, and comets. It doesn’t have to be aluve to be deeply informative about the universe and our place in it – in facr I often find myself thinking that modern space exploration is a bit too focussed on searching for life. The universe is still amazing and worth exploring, even if we truly are alone.
I don’t believe “comparable chemistry” is what we can expect on Titan. The average temperature is reported to be in the 90s Kelvin. While on Earth it’s 280 something Kelvin. I doubt speed of chemical reactions scale linearly, but wouldn’t temperature be the main factor driving this process?
There is one compound that could be the backbone
of analogues to Carbons role in biochemistry on Titan. Silicone.
In temps above 0 C, Silicone forms Very unstable amino acid like chains. (and it’s only small retinue “amino acids” and like compounds neccesary for life on Earth that can form)
Two big problems with this (i’m sure there’s more)
A) there is no evidence of large amount silicone on the surface of Titan. (athough if Titan has cryovolcanoes I supposed it could spewed up from the deep ocean.
B) At -155C to -160C, it may be too cold for chemical reactions.
C) I have no idea if any reactions are possible in a liquid methane solvent(aqueous analogue) inside a cell membrane made of acrylonitrile
Here is a paper from 1997 by some Titan experts on what may happen in terms of life on that moon when Sol becomes a red giant:
http://www.lpl.arizona.edu/~rlorenz/redgiant.pdf
Another interesting relevant site:
http://www.titanexploration.com/lifetitan.htm
And this one from 2016:
http://www.exoclimes.com/topics/the-far-future-of-the-solar-system-titan-as-an-ocean-planet/
Was it though? Certainly it was geologically active, whether that geology involved plate tectonics is another matter.
APL proposes Dragonfly mission to explore potential habitable sites on Saturn’s largest moon
August 24, 2017
by Khadija Elkharbibi
The Johns Hopkins Applied Physics Laboratory has submitted a proposal to NASA outlining a daring New Frontiers-class mission concept that would use an instrumented, radioisotope-powered dual-quadcopter to explore potential habitable sites where life could be developed on Saturn’s largest moon, Titan.
“Seems pretty straightforward,” quipped Dragonfly project manager Peter Bedini in a video presentation of the proposal.
The concept, called Dragonfly, would capitalize on the rapid advances made in recent years to autonomous aerial systems—sometimes referred to as the “drone revolution.” The increased reliability and capability these systems would enable Dragonfly to make numerous flights, moving from one geologic setting to another on the moon’s surface.
Full article here:
https://phys.org/news/2017-08-apl-dragonfly-mission-explore-potential.html
To quote:
“This is the kind of experiment we can’t do in the laboratory because of the time scales involved,” said APL’s Elizabeth Turtle, principal investigator for the Dragonfly mission. “Mixing of rich, organic molecules and liquid water on the surface of Titan could have persisted over very long timescales. Dragonfly is designed to study the results of Titan’s experiments in prebiotic chemistry.”
http://spaceref.com/saturn/titan-has-extreme-methane-rainstorms.html
Titan Has Extreme Methane Rainstorms
Press Release – Source: UCLA
Posted October 12, 2017 8:58 PM
Titan, the largest of Saturn’s more than 60 moons, has surprisingly intense rainstorms, according to research by a team of UCLA planetary scientists and geologists.
Although the storms are relatively rare — they occur less than once per Titan year, which is 29 and a half Earth years — they occur much more frequently than the scientists expected.
“I would have thought these would be once-a-millennium events, if even that,” said Jonathan Mitchell, UCLA associate professor of planetary science and a senior author of the research, which was published Oct. 9 in the journal Nature Geoscience. “So this is quite a surprise.”
The storms create massive floods in terrain that are otherwise deserts. Titan’s surface is strikingly similar to Earth’s, with flowing rivers that spill into great lakes and seas, and the moon has storm clouds that bring seasonal, monsoon-like downpours, Mitchell said. But Titan’s precipitation is liquid methane, not water.
“The most intense methane storms in our climate model dump at least a foot of rain a day, which comes close to what we saw in Houston from Hurricane Harvey this summer,” said Mitchell, the principal investigator of UCLA’s Titan climate modeling research group.
Sean Faulk, a UCLA graduate student and the study’s lead author said the study also found that the extreme methane rainstorms may imprint the moon’s icy surface in much the same way that extreme rainstorms shape Earth’s rocky surface.
On Earth, intense storms can trigger large flows of sediment that spread into lowlands and form cone-shaped features called alluvial fans. In the new study, the UCLA scientists found that regional patterns of extreme rainfall on Titan are correlated with recent detections of alluvial fans, suggesting that they were formed by intense rainstorms.
The finding demonstrates the role of extreme precipitation in shaping Titan’s surface, said Seulgi Moon, UCLA assistant professor of geomorphology and a co-senior author of the paper. Moon said the principle likely applies to Mars, which has large alluvial fans of its own, and to other planetary bodies. Greater understanding of the relationship between precipitation and the planetary surfaces could lead to new insights about the impact of climate change on Earth and other planets.
Titan’s alluvial fans were detected by a radar instrument on the Cassini spacecraft, which began orbiting Saturn in late 2004. The Cassini mission ended in September 2017, when NASA programmed it to plunge into the planet’s atmosphere as a way to safely destroy the spacecraft.
Juan Lora, a UCLA postdoctoral scholar and a co-author of the paper, said Cassini has revolutionized scientists’ understanding of Titan.
Although Titan’s alluvial fans are a new discovery, scientists have had eyes on the moon’s surface for years. Shortly after Cassini reached Saturn, radar and other instruments showed that vast sand dunes dominated Titan’s lower latitudes, while lakes and seas dominated its higher latitudes. The UCLA scientists found that the alluvial fans are mostly located between 50 and 80 degrees latitude — close to the centers of the moon’s northern and southern hemispheres, but generally slightly closer to the poles than to the equator.
Such variations in surface features suggest the moon has corresponding regional variations in precipitation, because rainfall and subsequent runoff play a key role in eroding land and filling lakes, while the absence of rainfall promotes the formation of dunes.
Previous models have shown that liquid methane generally concentrates on Titan’s surface at higher latitudes. But no previous study had investigated the behavior of extreme rainfall events that might be capable of triggering major sediment transport and erosion, or shown their connection to surface observations.
The scientists primarily used computer simulations to study Titan’s hydrologic cycle because observations of actual precipitation on Titan are difficult to obtain and because, given the length of each year on Titan, Cassini only observed the moon for three seasons. They found that while rain mostly accumulates near the poles, where Titan’s major lakes and seas are located, the most intense rainstorms occur near 60 degrees latitude — precisely the region where alluvial fans are most heavily concentrated.
The study suggests that the intense storms develop due to the sharp differences between the wetter, cooler weather in the higher latitudes and the drier, warmer conditions in the lower latitudes. Similar temperature contrasts on Earth produce intense cyclones in the mid-latitudes, which is what creates the storms and blizzards that are common during the winter months across much of North America.
Reference: “Regional Patterns of Extreme Precipitation on Titan Consistent with Observed Alluvial Fan Distribution,” S. P. Faulk et al., 2017 Oct. 9, Nature Geoscience:
https://www.nature.com/articles/ngeo3043
The research was funded by a NASA Cassini Data Analysis and Participating Scientists Program grant.
The human race seems to be sinking to new lows every day, but then I am reminded that the species can also make detailed maps of an alien moon 800 million miles from Earth:
https://www.sciencenews.org/article/most-complete-map-titan-reveals-connected-seas-and-cookie-cutter-lakes
Atmospheric Circulation, Chemistry, and Infrared Spectra of Titan-like Exoplanets Around Different Stellar Types
Press Release – Source: astro-ph.EP
Posted December 12, 2017 9:37 PM
http://astrobiology.com/2017/12/atmospheric-circulation-chemistry-and-infrared-spectra-of-titan-like-exoplanets-around-different-ste.html
Why NASA’s Dragonfly Mission Could Be Big in the Hunt for Aliens
https://www.inverse.com/article/39626-nasa-new-frontiers-finalist-titan-dragonfly
To quote:
It’s also worth noting that one of the two concepts chosen to “receive technology development funds” is Enceladus Life Signatures and Habitability (ELSAH), which seems to focus on finding biosignatures on Saturn’s strangest moon. According to a press release from NASA, the mission seeks to “develop cost-effective techniques that limit spacecraft contamination and thereby enable life detection measurements on cost-capped missions.” As we know from NASA’s Cassini mission, Enceladus and its subterranean ocean seem to have all the right ingredients to harbor life.