At the last Tennessee Valley Interstellar Workshop, I was part of a session on biosignatures in exoplanet atmospheres that highlighted how careful we have to be before declaring we have found life. Given that, as Alex Tolley points out below, our own planet has been in its current state of oxygenation for a scant 12 percent of its existence, shouldn’t our methods include life detection in as wide a variety of atmospheres as possible? A Centauri Dreams regular, Alex addresses the question by looking at new work on chemical disequilibrium and its relation to biosignature detection. The author (with Brian McConnell) of A Design for a Reusable Water-Based Spacecraft Known as the Spacecoach (Springer, 2016), Alex is a lecturer in biology at the University of California. Just how close are we to an unambiguous biosignature detection, and on what kind of world will we find it?
by Alex Tolley

Image: Archaean or early Proterozoic Earth showing stromatolites in the foreground. Credit: Peter Sawyer / Smithsonian Institution.
The Kepler space telescope has established that exoplanets are abundant in our galaxy and that many stars have planets in their habitable zones (defined as having temperatures that potentially allow surface water). This has reinvigorated the quest to answer the age-old question “Are We Alone?”. While SETI attempts to answer that question by detecting intelligent signals, the Drake equation suggests that the emergence of intelligence is a subset of the planets where life has emerged. When we envisage such living worlds, the image that is often evoked is of a verdant paradise, with abundant plant life clothing the land and emitting oxygen to support respiring animals, much like our pre-space age visions of Venus.
Naturally, much of the search for biosignatures has focused on oxygen (O2), whose production on Earth is now primarily produced by photosynthesis. Unfortunately, O2 can also be produced abiotically via photolysis of water, and therefore alone is not a conclusive biosignature. What is needed is a mixture of gases in disequilibrium that can only be maintained by biotic and not abiotic processes. Abiotic processes, unless continually sustained, will tend towards equilibrium. For example, on Earth, if life completely disappeared today, our nitrogen-oxygen dominated atmosphere would reach equilibrium with the oxygen bound as nitrate in the ocean.
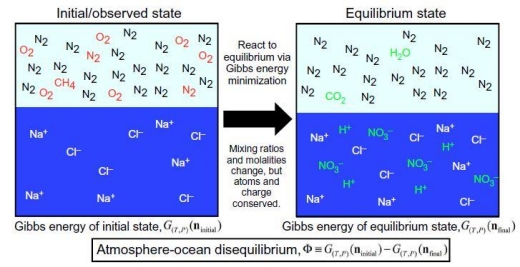
Image: Schematic of methodology for calculating atmosphere-ocean disequilibrium. We quantify the disequilibrium of the atmosphere-ocean system by calculating the difference in Gibbs energy between the initial and final states. The species in this particular example show the important reactions to produce equilibrium for the Phanerozoic atmosphere-ocean system, namely, the reaction of N2, O2, and liquid water to form nitric acid, and methane oxidation to CO2 and H2O. Red species denote gases that change when reacted to equilibrium, whereas green species are created by equilibration. Details of aqueous carbonate system speciation are not shown. Credit: Krissansen-Totton et al. (citation below).
Another issue with looking for O2 is that it assumes a terrestrial biology. Other biologies may be different. However environments with large, sustained, chemical disequilibrium are more likely to be a product of biology.
A new paper digs into the issue. The work of Joshua Krissansen-Totton (University of Washington, Seattle), Stephanie Olson (UC-Riverside) and David C. Catling (UW-Seattle), the paper tackles a question the authors have addressed in an earlier paper:
“Chemical disequilibrium as a biosignature is appealing because unlike searching for biogenic gases specific to particular metabolisms, the chemical disequilibrium approach makes no assumptions about the underlying biochemistry. Instead, it is a generalized life-detection metric that rests only on the assumption that distinct metabolisms in a biosphere will produce waste gases that, with sufficient fluxes, will alter atmospheric composition and result in disequilibrium.”
This approach also opens up the possibility of detecting many more life-bearing worlds as the Earth’s highly oxygenated atmosphere has only been in this state for about 12% of the Earth’s existence.
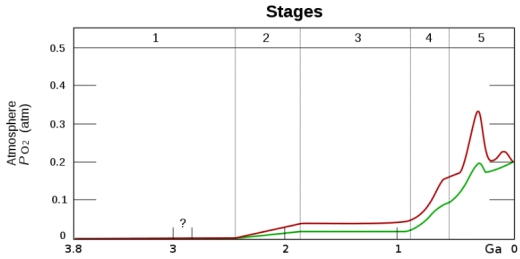
Image: Heinrich D. Holland derivative work: Loudubewe (talk) – Oxygenation-atm.svg, CC BY-SA 3.0,
https://commons.wikimedia.org/w/index.php?curid=12776502
With the absence of high partial pressures of O2 before the Pre-Cambrian, are there biogenic chemical disequilibrium conditions that can be discerned from the state of primordial atmospheres subject to purely abiotic equilibrium?
The new Krissansen-Totton et al? paper attempts to do that for the Archaean (4 – 2.5 gya) and Proterozoic (2.5 – 0.54) eons. Their approach is to calculate the Gibbs Free Energy (G), a metric of disequilibrium, for gases in an atmosphere-oceanic environment. The authors use a range of gas mixtures from the geologic record and determine the disequilibrium they represent using calculations of G for the observed versus the expected equilibrium concentrations of chemical species.
The authors note that almost all the G is in our ocean compartment from the nitrogen (N2)-O2 not reaching equilibrium as ionic nitrate. A small, but very important disequilibrium between methane (CH4) and O2 in the atmosphere is also considered a biosignature.
Using their approach, the authors look at the disequilibria in the atmosphere-ocean model in the earlier Archaean and Proterozoic eons. The geologic and model evidence suggests that the atmosphere was largely N2 and carbon dioxide (CO2), with a low concentration of O2 (2% or less partial pressure) in the Proterozoic.
In the Proterozoic, as today, the major disequilibrium is due to the lack of nitrate in the oceans and therefore the higher concentrations of O2 in the atmosphere. Similarly, an excess concentration of CH4 that should quickly oxidize to CO2 at equilibrium. In the Archaean, prior to the increase in O2 from photosynthesis, the N2, CO2, CH4 and liquid H2O equilibrium should consume the CH4 and increase the concentration of ammonium ions (NH4+ ) and bicarbonate (HCO3-) in the ocean. The persistence of CH4 in both eons is primarily driven by methanogen bacteria.
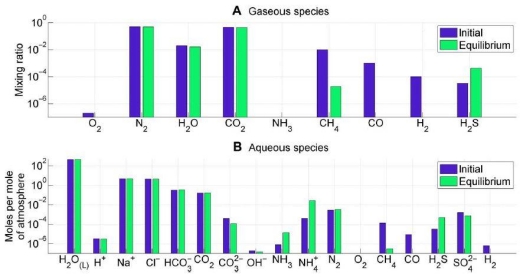
Image: Atmosphere-ocean disequilibrium in in the Archean. Blue bars denote assumed initial abundances from the literature, and green bars denote equilibrium abundances calculated using Gibbs free energy minimization. Subplots separate (A) atmospheric species and (B) ocean species. The most important contribution to Archean disequilibrium is the coexistence of atmospheric CH4, N2, CO2, and liquid water. These four species are lessened in abundance by reaction to equilibrium to form aqueous HCO3 and NH4. Oxidation of CO and H2 also contributes to the overall Gibbs energy change. Credit: Krissansen-Totton et al.
Therefore a biosignature for such an anoxic world in a stage similar to our Archaean era, would be to observe an ocean coupled with N2, CO2 and CH4 in the atmosphere. There is however an argument that might make this biosignature ambiguous.
CH4 and carbon monoxide (CO) might be present due to impacts of bolides (Kasting). Similarly, under certain conditions, it is possible that the mantle might be able to outgas CH4. In both cases, CO would be present and indicative of an abiogenic process. On Earth, CO is consumed as a substrate by bacteria, so its presence should be absent on a living world, even should such outgassing or impacts occur. The issue of CH4 outgassing, at least on earth, is countered by the known rates of outgassing compared to the concentration of CH4 in the atmosphere and ocean. The argument is primarily about rates of CH4 production between abiotic and biotic processes. Supporting Kasting, the authors conclude that on Earth, abiotic rates of production of CH4 fall far short of the observed levels.
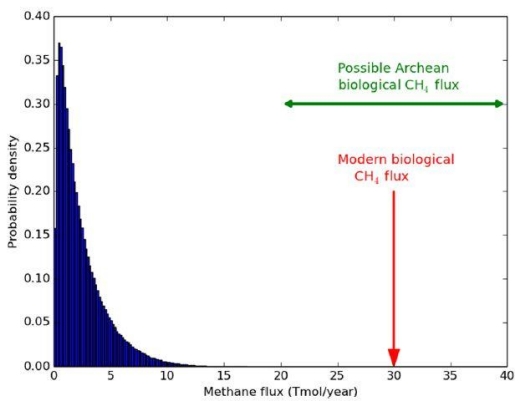
Image: Probability distribution for maximum abiotic methane production from serpentinization on Earth-like planets. This distribution was generated by sampling generous ranges for crustal production rates, FeO wt %, maximum fractional conversion of FeO to H2, and maximum fractional conversion of H2 to CH4, and then calculating the resultant methane flux 1 million times (see the main text). The modern biological flux (58) and plausible biological Archean flux (59) far exceed the maximum possible abiotic flux. These results support the hypothesis that the co-detection of abundant CH4 and CO2 on a habitable exoplanet is a plausible biosignature. Credit: Krissansen-Totton et al.
The authors conclude that their biosignature should also exclude the presence of CO to confirm the observed gases as a biosignature:
“The CH4-N2-CO2-H2O disequilibrium is thus a potentially detectable biosignature for Earth-like exoplanets with anoxic atmospheres and microbial biospheres. The simultaneous detection of abundant CH4 and CO2 (and the absence of CO) on an ostensibly habitable exoplanet would be strongly suggestive of biology.”
Given these gases in the presence of an ocean, can we use them to detect life on exoplanets?
Astronomers have been able to detect CO2, H2O, CH4 and CO in the atmosphere of HD 189733b, which is not Earthlike, but rather a hot Jupiter with a temperature of 1700F, far too hot for life. So far these gases have not been detectable on rocky worlds. Some new ground-based telescopes and the upcoming James Webb Space Telescope should have the capability of detecting these gases using transmission spectroscopy as these exoplanets transit their star.
It is important to note that the presence of an ocean is necessary to create high values of G. The Earth’s atmosphere alone has quite a low G, even compared to Mars. It is the presence of an ocean that results in G orders of magnitude larger than that from the atmosphere alone. Such an ocean is likely to be first detected by glints or the change in color of the planet as it rotates exposing different fractions of land and ocean.
An interesting observation of this approach is that a waterworld or ocean exoplanet might not show these biosignatures as the lack of weathering blocks the geologic carbon cycle and may preclude life’s emergence or long term survival. This theory might now be testable using spectroscopy and calculations for G.
This approach to biosignatures is applicable to our own solar system. As mentioned, Mars’ current G is greater than Earth’s atmosphere G. This is due to the photochemical disequilibrium of CO and O2. The detection of CH4 in Mars’ atmosphere, although at very low levels, would add to their calculation of Mars’ atmospheric G. In future, if the size of Mars’ early ocean can be inferred and gases in rocks extracted, the evidence for paleo life might be inferred. Fossil evidence of life would then confirm the approach.
Similarly, the composition of the plumes from Europa and Enceladus should also allow calculation of G for these icy moons and help to infer whether their subsurface oceans are abiotic or support life.
Within a decade, we may have convincing evidence of extraterrestrial life. If any of those worlds are not too distant, the possibility of studying that life directly in the future will be exciting.
The paper is Krissansen-Totton? et al., “Disequilibrium biosignatures over Earth history and implications for detecting exoplanet life,?” (2018)? Science Advances 4 ? (abstract? / ?full tex?t).



I want to draw your attention to this paper that was posted by ljk in the comment thread of the Zubrin article.
SPECTRA OF EARTH-LIKE PLANETS THROUGH GEOLOGICAL EVOLUTION AROUND FGKM STARS It is relevant to this post as it simulates the spectra of an earthlike planet during different geological eons orbiting different stellar types. The paper goes into detail over a range of potentially detectable gases and how the star’s emission temperature can affect gas ratios and detectable spectra. I think it helps elaborate on the key findings of the posted paper and leads the way forward regarding biosignature detection, especially during the Archaen and early Proterozoic where O2 levels are very low or effectively absent.
A new way to detect, not JUST bioSIGNATURES, but UNIMPEACHABLE evidence of algae-like and plankton-like life on an exoplanet has just been put forward! Earth’s ionosphere is litterally PACKED with single oxygen ions, and hardly anything else. Only PHOTOSYNTHESIS can accomplish this feat. Of course, on Earth, flowering plants and tree forests also contribute a small fraction of these ions, but the GREAT MAJORITY is produced by algae and plankton. Unfortunately, it will be AT LEAST TEN YEARS before we have a telescope that can detect an exoplanet’s ionosphere, and in that time, I am sure scientists will come up with alternate explanations for such a signal, but I SERIOUSLY DOUBT if such explanations will hold up to scrutiny.
Harry, do you have a link for that?
https://www.nature.com/articles/s41550-017-0375-y
Of course I will publish a paper with ‘alternative explanations’ if a study leave a hole large to park my car in!
That’s how good science work.
If there is no follow up then there’s is time to worry, it is deemed uninterested, or to crazy and left safely ignored.
A the paper then have fewer quotations, will soon be forgotten.
More who publish is to be preferred, and good for science.
On the weathering of land and the carbon cycle….
Would a frozen world be able to produce enough weathering with glaciers or other frozen formations?
I think the answer is no. The hypothesized snowball condition of the Earth before the Cambrian Explosion was melted by volcanic emissions of greenhouse gases that warmed the Earth. Isn’t the frozen Antarctic a low weathering example?
As for the Archaean eon, planetologists have been trying to determine what gases would be needed to keep the Earth’s surface unfrozen while the sun was much dimmer. Kasting has even suggested H2 was needed beyond CO2 and CH4. Hence the idea that maybe life started at ocean vents where heat and chemistry were available, rather than in a warm pond on the surface as Darwin postulated.
Yes, a frozen world with ice and snow could very well have weathering and might also have oxygen and could result in a false positive.
I like the idea of looking for exoplanets which might resemble Earth in different epochs since the possibilities are there. The emission of FGKM stars is in the visual and infra red spectrum and these stars have life belts at different distances which is dependent of the temperature, brightness, mass etc.
There is less atmosphere above the troposphere and cloud tops but there should still be detectable heavy gases like Co2 and Ch4 in the stratosphere of exoplanets with spectroscopy especially the light polarization technique. One thing not mentioned here is ozone. Life needed ozone to block the ultra violet light. Molecular oxygen O2 becomes split apart by ultra violet light and becomes atomic oxygen O and combines with O2 to become ozone, O3. This is what happened early on Earth when oxygen levels increased so there was built of a ozone shield harmful UV radiation. There is much more ozone in an atmosphere where there is a substantial amount of oxygen which is replenished by photosynthesis. CH4 also destroys ozone and these can only be there on a geological time scale with life produced oxygen. CH4 does not hang around very long and has to be replenished so you can bet if it is there with the other bio signature gases it might indicate life. Consequently, the spectral signature should also include Ozone as well as CH4, H20, N and Oxygen. Nitrogen also follows a cycle which is supported and replenished by life.
Clouds also have a spectral signature in the blue and near ultra violet. The Earth is thought to be a very cloudy world when the Co2 levels were much higher 250 million years ago than today but there still would be some cloudless openings and a clear, blue sky there where continual observations might yield lower atmospheric spectrum. H20 should be easily detected in the near infra red and mid infra red.
At the surface of the ocean and on land. If the UV remains high, then life might be restricted to the rocks and the oceans well below the surface.
Some new ground-based telescopes and the upcoming James Webb Space Telescope should have the capability of detecting these gases using transmission spectroscopy as these exoplanets transit their star.
what are these new ground based telescopes?
This article seems relevant to the discussion but needing more expertise than I can offer:
https://link.springer.com/article/10.1007/s10910-012-9977-x
The concept of network returnability is reformulated as an equilibrium constant for a reaction network. Using this concept we study the atmospheric reaction networks of Earth, Mars, Venus and Titan. We found that the reaction network in the Earth’s atmosphere has the largest disequilibrium, followed by that of Titan…
The suggestion that Titan has a high level of atmospheric disequilibrium, second only to Earth, appears intriguing.
It seems that the statement from the Estrada paper is opposite to that of the Disequilibrium paper, which puts Titan at a very low disequilibrium. I don’t know what “Returnability” is, but I have downloaded the paper to try to understand what the authors have done.
The most relevant bits from the Returnability paper:
We now turn our attention to Titan, which has the atmosphere with the second least returnable reaction network. This satellite has some characteristics that place it in a unique position in our solar system. It has liquid at the surface, a thick atmosphere, energy sources such as energetic electrons and solar UV, has complex chemical reactions, precipitation, erosion, volcanism, impact processing, etc. All of which makes it similar to the primitive Earth. It has an atmosphere with a reaction network which is 10 times more returnable than that of the Earth, but still 10 times less returnable than those of Venus and Mars.
Earth pKr: 3.672
Titan pKr: 2.796 = 7.5 times as returnable as Earth
Mars pKr: 1.787 = 10.2 times as returnable as Titan
This is no surprise. Titan has been known for several year to have an atmosphere in disequilibrium, in ways that had actually been predicted beforehand under the assumption that bacterial-grade metabolisms exists on Titan’s surface.
The prediction was for a sink for molecular hydrogen at the surface, together with absence of acetelyne at the surfce (should otherwise be a lot, generated through photochemical reactions and settling to the surface, and an excess of methane, which should otherwise be destroyed by photochemical reactions.
This is also what Cassini measurements found — no acetelyne on the surface, a molecular hydrogen sink at the surface, and way too much methane in the atmosphere, implying a steady production rate from somewhere.
The proposed metabolism uses molecular hydrogen and sunlight, and produces methane as a waste product. High-energy compounds like acetelyne are also consumed when they would otherwise just sit there in meters-deep drifts.
It is an interesting hypothesis, but the evidence is rather thin. Life is more than metabolism (combustion), so we would need other evidence before we accepted that any disequilibrium was caused by life of some kind. It would certainly be exciting if such life existed as it would be very different from terrestrial life.
what are these new ground based telescopes?
From the introduction of the paper:
I’m sureour resident telescope experts can provide a lot more details.
Quotes by Alex Tolley:
Given these gases in the presence of an ocean, can we use them to detect life on exoplanets? Yes if it is in the Archean period before the oxygen catastrophe where oxygen reduced the levels of methane. If not one could still assume it was an Earthlike planet in the early Archean before the oxygen catastrophe. There still was some oxygen in the Proterozoic.
The problem with the ocean on Mars is that when it had a thicker atmosphere, the brightness of the Sun was less. It might have been mostly always frozen or not been around long enough for life to have started. Scientists have considered Mars early in its history to have a higher pressure over half of Earths but it might not have lasted due to atmospheric escape caused by the solar wind sputtering and heavy meteorite bombardment.
Also there is the snowball Earth period which had less Co2 followed by the hottest period in history with a lot of volcanic out gased Co2. The rain takes the Co2 out of the atmosphere due to the Urey reaction and volcanism and plate subduction put it back into the air through the carbon cycle. It is clear that life has affected the atmospheric Co2 since there will be no more snow ball Earth periods and there were no ice ages and polar ice caps over four million years ago since the Co2 levels have been gradually reduced by less volcanism and Co2 removing plants etc. The Sun has become brighter also so our biosphere has become dependent on less atmospheric Co2 and a stable carbon cycle and volcanism.
The spectra of a snow ball Earth might be easier to detect since the atmosphere would be less cloudy, but a hot greenhouse period might have had much more Co2 100 to 10,000 times today’s Co2 which would be have a more cloudy atmosphere. There would be no polar ice caps, more water vapor in the atmosphere so the storms or hurricanes would be stronger due to more energy in the system and warm air holds more water vapor and more moisture for clouds and heat for storms. Cold air holds less water vapor.
“The authors conclude that their biosignature should also exclude the presence of CO to confirm the observed gases as a biosignature:” I agree it would be suggestive of biology. I wonder why it excludes the presence of CO? It there some kind of chemical reactions than remove CO? The CO of course in our spectral signature would indicate the man made burning of fossil fuels if we include the biosignature gases in the spectra.
Most of the Co2 is locked up in the rocks as CO3 due to weathering and also marine organisms like coccolithophores.
The impact of comets brings CH4, H2O, CO2, and CO. Therefore these gases might represent a false positive. CO would be removed over time by life, so it must be absent for the biosignature.
The take-home message from the paper is that high O2 partial pressures occurred quite late in Earth’s history, yet life was present from quite early. This means that looking for O2 as part of a biosignature is leaving out the majority of planets that may be living, but where photosynthesis has not had time to build up the atmospheric levels of O2. Biosignatures that work for planets before their GOE should find more planets than ones with an O2 focus.
The authors of this paper, and the authors of the “SPECTRA…” paper assume an Earth-like world, which to my understanding includes one large enough to retain an atmosphere. Early Mars might have had life (it is one reason Nasa continues to study Mars in such depth for signs of water and life – current or fossil) but if it loses it atmosphere, even if life survives as lithophilic bacteria (and maybe the source of CH4), the thin atmosphere makes it hard to obtain spectra at stellar distances. The focus of these papers is to propose models for candidate evaluation under different conditions where life may exist and be detectable today.
Human use of combustion technology is extremely recent and may even disappear in a similarly short time. A millennium of fossil fuel combustion represents just 0.000025% of the life era on Earth so far. This might represent the probability of detecting a technological civilization similar to ours. Far more likely we will detect non-technological life, and not even multicellular life. Advanced civilizations even if they are millions or even billions of years old will be very different technologically from us and probably not inefficiently be combusting carbon fuels. If we detect CO in an otherwise O2 atmosphere, I would speculate this is the result of global forest fires from a KT-like impact, rather than the output of technology. Looking for chlorofluorocarbons might settle the matter, as these are purely artificial compounds on Earth.
On the other hand a thicker atmosphere would make the ocean have a better chance of existing in a liquid state. Also a plus half a bar atmosphere might shield some of the ionizing gamma and x-ray radiation which is unblocked in today’s thin Martian atmosphere so life could have evolved in an early Mars atmosphere. There are some more harder species microbes or archaea which might survive a UV environment. There might have been some ozone if there was more oxygen.
The earlier Krissansen-Totton paper suggests Mars has the most disequilibrated atmosphere after Earth due to photolysis, and that Titan has a moderate disequilibrium due to higher ethane composition than ex[ected for unknown reasons.
The Estrada paper uses their “Returnability” index based on what appears to be disequilibrium of difference atmospheric components versus a null model of complete two-way reactions for all components. I am not clear why their approach makes sense compared to more standard equilibrium models. The result, however, is that Titan appears to have a high disequilibrium, rather like Earth.
Interesting post!
Can we sort of summarize it by stating that the main biosignatures on an earthlike planet are CH4 before oxygenation (Prokaryotic life: methanogenic bacteria), and O2/O3 after oxygenation (Eukaryotic life).
I wonder, however, whether there could be a (long) time period during which the CH4 producers have already been largely outcompeted by the O2 producers, and yet the O2 does not show up yet, because of absorption in the planet’s sinks (crust, upper mantle). In other words, a period with very little biosignature/disequilibrium at all. Somewhere during the Proterozoic?
Broadley, yes, although there must be evidence of disequilibrium and other features to try to rule out false positives. For example the need for absence of CO to rule out an abiotic atmosphere due to cometary impacts alone, and a disequilibrium gas like CH4 or N2 during the high atmospheric O2 era of modern Earth.
AFAIK, that is the case. The snowball Earth may also have contributed to this by reducing photosynthetic O2 production. However, according to the authors, disequilibrium is maintained throughout the Proterozoic. The authors suggest that with just the atmosphere and excluding the ocean compartment, disequilibrium declined after the Cambrian era. (See Figure 2 in the paper.) The authors stress that disequilibrium should include the ocean compartment and that the search for life should confirm surface oceans, perhaps by looking for ocean glint.
Quote by Alex Tolley:
“The authors of this paper, and the authors of the “SPECTRA…” paper assume an Earth-like world, which to my understanding includes one large enough to retain an atmosphere. Early Mars might have had life (it is one reason Nasa continues to study Mars in such depth for signs of water and life – current or fossil) but if it loses it atmosphere, even if life survives as lithophilic bacteria (and maybe the source of CH4), the thin atmosphere makes it hard to obtain spectra at stellar distances. The focus of these papers is to propose models for candidate evaluation under different conditions where life may exist and be detectable today.
The CO of course in our spectral signature would indicate the man-made burning of fossil fuels if we include the biosignature gases in the spectra.
Human use of combustion technology is extremely recent and may even disappear in a similarly short time. A millennium of fossil fuel combustion represents just 0.000025% of the life era on Earth so far. This might represent the probability of detecting a technological civilization similar to ours. Far more likely we will detect non-technological life, and not even multicellular life. Advanced civilizations even if they are millions or even billions of years old will be very different technologically from us and probably not inefficiently be combusting carbon fuels. If we detect CO in an otherwise O2 atmosphere, I would speculate this is the result of global forest fires from a KT-like impact, rather than the output of technology. Looking for chlorofluorocarbons might settle the matter, as these are purely artificial compounds on Earth.”
Absolutely, I agree. The millennium of burning fossil fuels will have to include period before 1850 and the industrial revolution since I doubt there will be any after 300 years from today since all automobiles will be electric or hydrogen fuel cells since there is only 75 to 100 years of oil left. Even one’s hot water and the laundomatts will use electricity to heat clothes due to greenhouse gases emission control and the increasing cost of a dwindling source of non renewable energy which will astronomical by then, so CO spectra might even be seen for a less time than a 1000 years. I thought it was just interesting to know that a civilization at our level of technological development might be found. If its several hundred light years away we’d be seeing it in the past and not how it might look in the present.
Some exoplanets like those of Trappist One might never show an oxygen spectrum due to too much x-ray and UV radiation which is why life might be limited to G class stars. Exoplanets on class M stars are too close to the stars so they get a big dose of UV, X-ray and solar wind atmospheric degassing. A class stars have a shorter life on the main sequence only one billion years so life will never have time to evolve there; The life belt won’t remain stable and move away from the planet too early so the star type may be crucial.
We have yet to confirm the effects of a lack of magnetic field and solar wind degassing of an exoplanet atmosphere. Hopefully we will find out shortly. I hope we search for a lot more G and K class stars for exoplanets.
But how long did it take large brain life to develop here? If you look at Earth as not the perfect place for life to develop, then a perfect planet may jump from early micro life to something like the Cambrian explosion very quickly. When did large brains appear, after the dinosaurs were wiped out, so could evolution also jump from fish to something like mammals directly? What were the first large brain mammals, Elephants, whales, dolphins or some other species that disappeared or never made it past the size of a cat’s brain? The 500 million years that is stable in A class stars may be enough time and there has been many more A class stars over the life of our galaxy. So these civilizations that could have developed on the planets around A class stars would have to of developed interstellar travel or some other method to circumnavigate their imminent doom of their star.
Speaking of cats and dogs, they been watching us for millenniums, how long after we commit suicide would it take them to develop civilization. ;-)
The early development of earth’s atmosphere may have been heavily influenced by inductive heating caused by the young sun super flares and the earth’s weak magnetic field.
“NASA’s Kepler mission found stars that resemble our sun about a few million years after its birth. The Kepler data showed many examples of what are called “superflares” – enormous explosions so rare today that we only experience them once every 100 years or so. Yet the Kepler data also show these youngsters producing as many as ten superflares a day.
While our sun still produces flares and CMEs, they are not so frequent or intense. What’s more, Earth today has a strong magnetic field that helps keep the bulk of the energy from such space weather from reaching Earth. Space weather can, however, significantly disturb a magnetic bubble around our planet, the magnetosphere, a phenomenon referred to as geomagnetic storms that can affect radio communications and our satellites in space. It also creates auroras – most often in a narrow region near the poles where Earth’s magnetic fields bow down to touch the planet.
Our young Earth, however, had a weaker magnetic field, with a much wider footprint near the poles.”
https://www.nasa.gov/feature/goddard/2016/nasa-solar-storms-may-have-been-key-to-life-on-earth
With the magnetic reconnection plus the energy pump in from CME and super flares there may be rouge Giant pulses (GP).
“The electromagnetic tornado scheme in the polar vacuum gap not only realizes the origin of a circular polarization in GP but also supports the idea that radio emission appears in the pulsar internal vacuum
gap which is a cavity-resonator (with respect to the radio-frequency radiation) excited by particles accelerated in a longitudinal electric field in the gap. GPs localization near the wave-guides and slots
means that the magnetosphere in the region of open field lines is basically not transparent for radiation except for the localization places. Energy emission through the breaks existing or accidentally appearing
in the magnetosphere of open field lines corresponds to giant pulses. Extremely high energy density of GPs of order of 1015 erg/cm3
seems to be a key moment.
It will be noted that trying to explain the GPs by strongly nonlinear effects in magnetosphere plasma where different variants of two-stream instability are realized requires considering such mechanisms as
modulation instability, Zakharov plasma wave collapse (the more popular!), reconnection of magnetic field lines, induced scattering in narrow beams etc. GP phenomena resemble also those of “rogue” or “freak” waves in nonlinear hydrodynamics of sea waves. Both that point of view and the above-stated may exist not interfering each other.”
Quantized electromagnetic tornado in pulsar vacuum gap.
https://arxiv.org/ftp/arxiv/papers/0909/0909.1018.pdf
I’M MELTING! —
Star’s magnetic field could turn habitable-zone planets into magma soup.
https://arstechnica.com/science/2017/10/stars-magnetic-field-could-turn-habitable-zone-planets-into-magma-soup/
Magma oceans and enhanced volcanism on TRAPPIST-1 planets due to induction heating.
https://arxiv.org/abs/1710.08761
Could this still be happening on Earth today???
November 18, 1882 – The Transit of Venus Storm – It produced a compass bearing deflection of nearly 2 degrees, All telegraphic transactions east of the Mississippi River and north of Washington D.C came to a halt. The Chicago stock market was severely affected all day. A large sunspot was then seen covering an area of more than three thousand millions of square miles. Simultaneously with the appearance of the spot, magnetic disturbances at the observatory in Greenwich increased in frequency and violence, other symptoms were noticed throughout the length of the British Isles. Telegraphic communication was greatly interfered with. The signal bells on many of the railway lines were rung, and some of the operators received shocks from their instruments. Lastly, on November 17, a superb aurora was witnessed, the culminating feature of which was the appearance, at about six o’clock in the evening, of a mysterious beam of greenish light, in shape something like a cigar, and many degrees in length, which rose in the east and crossed the sky at a pace much quicker than but nearly as even as that of sun, moon, or stars, till it set in the west two minutes after its rising. The daily press was burdened with accounts of widespread magnetic disturbance, in some places telegraphic communication was suspended. In Milwaukee the carbons in the electric lamps were lighted, rendered incandescent by currents of electricity flowing on the wires. At other locations, switchboards in telegraph offices were set on fire and sending keys were melted, while electric balls were seen hovering on the telegraph in Nebraska. [Newspaper Archive]
Nine months later the Krakatoa volcano exploded!
1883 eruption of Krakatoa
The most notable eruptions of Krakatoa culminated in a series of massive explosions over August 26–27, 1883, which were among the most violent volcanic events in recorded history.
Atmospheric Shock Waves Recorded Throughout the World
Atmospheric pressure shock waves from the explosions of Krakatoa circled the earth seven times and were recorded by barographs throughout the world. Barographic records documented the shock waves from the paroxysmal explosion of Krakatoa by as many 7 times, as these waves bounced back and forth between the site of the eruption site and its antipodes on the earth for 5 days following the explosion.
Upper Atmosphere Effects
Ash from the eruptions was propelled to a height of 50 miles (80 kilometers) in the upper atmosphere blocking the sun and plunging the surrounding region into darkness for two and a half days.
Dust and ash from the eruption encircled the Earth in 13 days forming a cloud which, by September 9, 1883, had covered completely the upper atmosphere along a belt in the equatorial zone. Three months after the eruption this belt of volcanic dust of fine particles of dust had spread to higher latitudes causing unusually spectacular red sunsets and other interesting atmospheric effects. Blue and green suns were also observed. Breathtaking sunsets were observed during the winter months of 1884 in both American and Europe. Unusual sunsets continued for almost 3 years.
Climatic Changes
It has been estimated that at least 21 cubic Km (appr. 11 cubic mile) was ejected from the eruption of Krakatoa and that at least 1 cubic mile of the finer material was blown to a height of about 17 miles (27 Km). The volcanic dust blown into the upper atmosphere was carried several times around the earth by air currents. This volcanic dust veil not only created the spectacular atmospheric effects described previously but acted also as a solar radiation filter, reducing the amount of sunlight reaching the surface of the earth. In the year following the eruption, global temperatures were lowered by as much as 1.2 degree Centigrade on the average. Weather patterns continued to be chaotic for years and there were major climatological changes which affected the entire globe. Temperatures did not return to normal until five years later, in 1888.
Alex:
Great post! My question to you is this: assuming we detect the signs of atmospheric disequilibrium you associate with life around an earthlike planet in our galactic neighborhood, then what values for Fl (abiogenesis) might we be able to assign to the Drake equation?
We need statistical data for this. I would then assign the probability of a planet having life based on this statistic.
However, that isn’t quite the question you asked. My bias is to then interpret this probability as abiogenesis, perhaps using Bayesian stats. However, it seems possible that a fraction of these worlds might be living due to panspermia. [Whether natural or directed is another issue]. I have generally thought panspermia, if it occurs, between star systems as extremely rare, but it may not be. The only way we can distinguish between panspermia and genesis is to send probes to those worlds and study the biology. That is a very long way off, and sadly, I will be long gone by then. For biologists studying life on those worlds, it will be a golden age, as each world will have evolved its own bisophere and unique organisms. With thousands or millions of planets’ biospheres catalogued, we could answer a host of questions about biology that are imposible today with our single example of Earth.
With a little luck, we may at least get an idea about the genesis vs panspermia debate in our own system if we discover life, living or fossilized, on other worlds.
I have a feeling that confirming biomarkers is going to be a lot harder than we thought. I was thinking how the Oxygen and Nitrogen components could change in Trappist planets depending on their composition.
Take inner Trappist planets. If they have a steam atmosphere over high pressure ice, then you could get a build up of Oxygen due to photo disassociation and Nitrogen oxidized to Nitric acid/Nitrogen dioxide. If the steam atmosphere was over magma, the Oxygen would be soaked up and you’d have Nitrogen in the atmosphere.
If the outer Trappist planets consisted of a deep ocean over high-pressure ice with no, or very little contact with the mantle, then again you could get a build up of Oxygen through photo disassociation, but no Nitrogen as it would all be converted to soluble Nitrates. If, however, the ocean was in contact with the mantle, then this would act as a sink for the Oxygen and you would get Nitrogen in the atmosphere.
We may be able to determine a lot about a planets internal composition just by looking at its atmospheric constituents.
I tend to agree that there will be all sorts of complications, creating both false positives and false negatives. Science is rarely definitive in its answers, just opening up avenues to more questions.
So, the detection of biosignatures on a planet, say, 50 light years away cannot be taken as evidence of an independent genesis because it could be the result of interstellar panspermia?
I see 3 main explanations for life:
1. abiogenesis
2. panspermia/lithospermia by natural causes
3. panspermia related to intelligence.
Let us assume a millennium from now we have analyzed life from a dozen or so planets.
If the biologies of those planets are very different, with just small overlaps, then I would theorize this is due to abiogenesis.
If the biologies are the same, or very similar, then I would tend to favor panspermia.
How to determine is panspermia was natural or intelligence directed? A model of natural panspermia might be that life arrives at random points in time. If so, all planets with the same biology might show the earliest life very early in their histories if the life transfer process was of high probability, or randomly starting in their histories if low probability. But supposing the fossil evidence and molecular clock evidence narrowed the start time to a few million years irrespective of the planet’s age? Then I might favor some sort of intelligence directed panspermia, terraforming or colonization program. Of course, all 3 processes could be happening that would muddy the picture.
A wider sample in space might show pockets of distinct biologies spread out in surrounding systems, suggesting points of life initiation that spread by some means of panspermia to nearby systems. It is even possible that we could find multiple biologies on the same planet, all coexisting.
There will clearly be plenty of room for theorizing and arguing at conferences!
One thing I have always wondered about. If life on Earth disappeared tomorrow, how long would the atmosphere take to return to equilibrium?
Also, during the snow ball Earth periods, how close to equilibrium would the atmosphere get?
Snowball earth does not show large changes in atmospheric gases. There is an increase in CO2 from volcanic eruptions and reduced carbon fixation, but the Earth never gets far towards equilibrium.
And this just in with regard to Proxima Centauri:
https://arxiv.org/abs/1802.08257
https://www.sciencedaily.com/releases/2018/02/180226103341.htm
“The flare increased Proxima Centauri’s brightness by 1,000 times over 10 seconds” and “A flare 10 times larger than a major solar flare would blast Proxima b with 4,000 times more radiation than the Earth gets from our Sun’s flares”.
Well, we already knew that about (many) M dwarfs. Not very favorable for (higher) life.
Atmosphere stripping may even make life detection of atmospheric gas spectra impossible to detect from Earth, creating a false negative.
The paper estimates that the N2/O2 atmosphere would equilibrate to nitrates in 20-200 million years.
The Detectability of Earth’s Biosignatures
Across Time.
Enric Palle
“Over the past two decades, enormous advances in the detection of exoplanets have taken place. Currently, we have discovered hundreds of earth-sized planets, several of them within the habitable zone of their star. In the coming years, the efforts will concentrate in the characterization of these planets and their atmospheres to try to detect the presence of biosignatures. However, even if we discovered a second Earth, it is very unlikely that it would present a stage of evolution similar to the present-day Earth. Our planet has been far from static since its formation about 4.5 Ga ago; on the contrary, during this time, it has undergone multiple changes in it’s atmospheric composition, it’s temperature structure, it’s continental distribution, and even changes in the forms of life that inhabit it. All these changes have affected the global properties of Earth as seen from an astronomical distance. Thus, it is of interest not only to characterize the observables of the Earth as it is today, but also at different epochs. Here we review the detectability of the Earth’s globally-averaged properties over time. This includes atmospheric composition and biosignatures, and surface properties that can be interpreted as sings of habitability (bioclues). The resulting picture is that truly unambiguous biosignatures are only detectable for about 1/4 of the Earth’s history. The rest of the time we rely on detectable bioclues that can only establish an statistical likelihood for the presence of life on a given planet.”
https://arxiv.org/abs/1802.09367
Thanks for the link. There seems to be a small spate of such papers. I look forward to reading it.
Very interesting and telling is Fig. 4.
And I quote: “Still the presence of O2 (or O3 as a proxy) in combination with large amounts of H2O vapor and CH4 in the Earth’s atmosphere provides the only true realistic biosignature susceptible to be used in the search for life on exoplanets, the so called “triple fingerprint”.
What we see in Fig. 4 is indeed this: the ‘true biosignature’ of O2/O3 (plus H2O and a little CH4) is present on Earth only during the later Proterozoic and the Phanerozoic. I would say that is a bit more than 1/4, but not more than about 1/3 of Earth’s history.
In other words: for the first 3 gy or so, it will be very hard to detect life on an Earth analogue.
I disagree with the the author’s conclusion. Pre-GOE biosignatures are quite doable based on the spectral work by those doing it. I have seen spectra that includes identification of all the molecules needed for the Archean/Proterozoic biosignatures.
Chemical Sleuthing Unravels Possible Path To Forming Life’s Building Blocks In Space
http://astrobiology.com/2018/03/chemical-sleuthing-unravels-possible-path-to-forming-lifes-building-blocks-in-space.html
How Earth’s Earliest Lifeforms Protected Their Genes – ScienceBlog.com
https://scienceblog.com/499693/earths-earliest-lifeforms-protected-genes/
Diatoms.
“The glass frustule allows light to pass through so that photosynthetic pigments can capture light energy for photosynthesis -UV protection? -Aid in sinking? -Protection from predation?”
http://images.slideplayer.com/17/5358443/slides/slide_2.jpg
https://en.wikipedia.org/wiki/Diatom#/media/File:Diatom2.jpg
Am assuming direct detection of magnetic fields isn’t yet in our tools palette, but can disequilibrium data be used to infer it via e.g. some sort of isotopic spectra? I don’t know if the life signature would be useful if there isn’t magnetic shielding, hence the question. What is known at this point about what sort of life earth would have without its magnetic field vs the expected disequilibrium footprint? And how would this impact any interpretation of what is seen?
Brewing up Earth’s earliest life
Researchers have found that a class of molecules called sulfidic anions may have been abundant in Earth’s lakes and rivers.
Large concentrations of sulfites and bisulfites in shallow lakes may have set the stage for Earth’s first biological molecules.
Jennifer Chu | MIT News Office
April 8, 2018
Around 4 billion years ago, Earth was an inhospitable place, devoid of oxygen, bursting with volcanic eruptions, and bombarded by asteroids, with no signs of life in even the simplest forms. But somewhere amid this chaotic period, the chemistry of the Earth turned in life’s favor, giving rise, however improbably, to the planet’s very first organisms.
What prompted this critical turning point? How did living organisms rally in such a volatile world? And what were the chemical reactions that brewed up the first amino acids, proteins, and other building blocks of life? These are some of the questions researchers have puzzled over for decades in trying to piece together the origins of life on Earth.
Now planetary scientists from MIT and the Harvard-Smithsonian Center for Astrophysics have identified key ingredients that were present in large concentrations right around the time when the first organisms appeared on Earth.
Full article here:
http://news.mit.edu/2018/earths-first-biological-molecules-0409
To quote:
Interestingly, he consulted the literature in a rather unexpected subject while conducting these calculations: winemaking — a science that involves, in part, dissolving sulfur dioxide in water to produce sulfites and bisulfites under oxygenless conditions similar to those on early Earth.
“When we were working on this paper, a lot of the constants and data we pulled out were from the wine chemistry journals, because it’s where we have anoxic environments here on modern Earth,” Ranjan says. “So we took aspects of wine chemistry and asked: ‘Suppose we have x amount of sulfur dioxide. How much of that dissolves in water, and then what does it become?’”
Community cross-talk
Ultimately, he found that, while volcanic eruptions would have spewed huge quantities of both sulfur dioxide and hydrogen sulfide into the atmosphere, it was the former that dissolved more easily in shallow waters, producing large concentrations of sulfidic anions, in the form of sulfites and bisulfites.
“During major volcanic eruptions, you might have had up to millimolar levels of these compounds, which is about laboratory-level concentrations of these molecules, in the lakes,” Ranjan says. “That is a titanic amount.”
The new results point to sulfites and bisulfites as a new class of molecules — ones that were actually available on early Earth — that chemists can now test in the lab, to see whether they can synthesize from these molecules the precursors for life.
Early experiments led by Ranjan’s colleagues suggest that sulfites and bisulfites may have indeed encouraged biomolecules to form. The team carried out chemical reactions to synthesize ribonucleotides with sulfites and bisulfites, versus with hydrosulfide, and found the former were able to produce ribonucleotides and related molecules 10 times faster than the latter, and at higher yields. More work is needed to confirm whether sulfidic anions were indeed early ingredients in brewing up the first life forms, but there is now little doubt that these molecules were part of the prebiotic milieu.
For now, Ranjan says the results open up new opportunities for collaboration.
“This demonstrates a need for people in the planetary science community and origins-of-life community to talk to each other,” Ranjan says. “It’s an example of how cross-pollination between disciplines can really yield simple but robust and important insights.”