I can remember when I first read about the experiment that Stanley Miller and Harold Urey performed at the University of Chicago in 1952 to see if organic molecules could be produced under conditions like those of the early Earth. It was a test of abiogenesis, though that wasn’t a word I knew at the time. Somewhere around 5th grade, I was a kid reading a book whose title has long escaped me, but the thought that scientists could re-create the atmosphere the way it was billions of years ago seized my imagination.
Never mind that exactly what was in that atmosphere has been controversial. What thrilled me was the attempt to reproduce something long gone — billions of years gone — and to experiment to find out what it might produce. I just finished Samanth Subramanian’s elegant biography of J. B. S. Haldane, the polymathic geneticist, mathematician, physiologist (and too much more to list here), whose work on the chemical formation of life was strongly supported by the Miller and Urey results, as was that of the Soviet biochemist Alexander Ivanovich Oparin, to whom Haldane always deferred when asked who should be given priority for the idea.
The biography, A Dominant Character (W. W. Norton, 2020) is a gem; I highly recommend it to those interested in these matters. And it was just the thing to be reading when I began to hear about the work of Fabian Schulz and Julien Maillard (IBM Research-Zurich).
Working with colleagues at the University of Paris-Saclay, the University of Rouen at Mont-Saint-Aignan, and the Fritz Haber Institute of the Max Planck Society, the researchers have been experimenting with atmospheres as well, though not of our own world but Titan, a moon frequently described as having analogues to the early Earth. In fact, they’ve re-created its atmosphere in an Earth laboratory, which may eventually tell us much about abiogenesis in both places through the use of atomic-scale microscopy.
Titan continues to fascinate. No other object in the Solar System offers up a nitrogen atmosphere of this density, along with organic processes and interactions between the atmosphere and the surface on a grand and highly visible scale. There is a distinct possibility that Earth’s atmosphere 2.8 billion years ago was close to what we see on Titan today. The timeframe is based on the creation of the first reef systems in the Mesoarchean Era, as cyanobacteria began their photosynthetic work to turn carbon dioxide into oxygen. So this is an obviously fecund arena for researchers to probe.
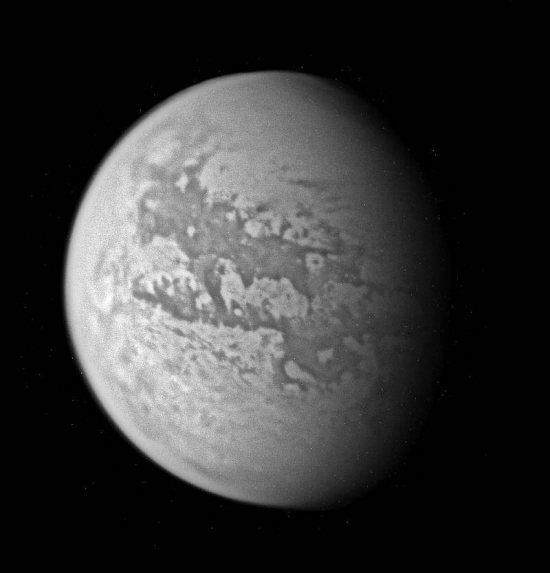
Image: Although the Huygens probe has now pierced the murky skies of Titan and landed on its surface, much of the moon remains for the Cassini spacecraft to explore. Titan continues to present exciting puzzles. This view of Titan uncovers new territory not previously seen at this resolution by Cassini’s cameras. The view is a composite of four nearly identical wide-angle camera images. Credit: NASA/JPL/Space Science Institute.
We’d like to know a lot more about that frustrating photochemical haze that hid the surface of Titan when Voyager 1 took its jog at Saturn to get a look at the moon. Here we’re seeing nanoparticles made out of organic molecules, with carbon, hydrogen and nitrogen in abundance. All this is the result of radiation from the Sun as it streams into the methane and nitrogen mix making up the bulk of Titan’s atmosphere. Previous lab experiments have focused on the organic molecules called tholins to understand the chemical nature of the molecules from which the haze is ultimately derived.
The term ‘tholin’ was first used in a 1979 Nature paper co-authored by Carl Sagan and Bishun Khare, who would doubtless be thrilled to see how large a role they play in our analysis of material in the outer system. Tholins got a lot of public exposure, for instance, when New Horizons flew past 486958 Arrokoth in the outer Solar System. They’re thought to have accounted for its reddish color, and are in fact common in this distant region as solar UV and cosmic rays interact with organic compounds on icy bodies.
The IBM experiment was structured to allow Schulz and Maillard to observe tholins in the formation process. Co-authors Leo Gross and Nathalie Carrasco explain:
“We flooded a stainless-steel vessel with a mixture of methane and nitrogen and then triggered chemical reactions through an electric discharge, thereby mimicking the conditions in Titan’s atmosphere. We then analyzed over 100 resulting molecules composing Titan’s tholins in our lab at Zurich, obtaining atomic resolution images of around a dozen of them with our home-built low-temperature atomic force microscope.”

Image: Titan’s aerosol analogues as seen by Scanning Electron Microscopy. Credit Nathalie Carrasco.
The work is significant because it is revealing how compounds like those found in Titan’s haze are built by using atomic-scale microscopy. This is a deep look into chemical bonding and structure that goes well beyond previous techniques, and offers what appears to be a new astrobiological tool. The scientists believe their work can be turned toward the analysis of Titan’s methane cycle, which like Earth’s hydrological cycle moves between a gaseous and liquid state, producing the moon’s lakes and seas.
The IBM work confirms that Titan’s orange haze is primarily made up of nitrogen-containing polycyclic aromatic hydrocarbons, with chemical structures that are related to the ‘wettability” of the haze, a factor that determines whether the haze nanoparticles float on the moon’s hydrocarbon lakes. From an IBM research blog:
Finding these new details on the chemical structure of tholins adds to our understanding not only of Titan’s haze but also of the likelihood that aerosols might have favored life on the early Earth in the past.
Did hazes like this at one time protect fragile DNA molecules from the Sun’s radiation? Gross and Carrasco point to the fact that the molecular structures the team has imaged are good absorbers of ultraviolet light. That would be useful information not just about the early Earth but also about the prospects for forms of life emerging on Titan itself. Future missions like Dragonfly should give us much information in this regard.
Meanwhile, I’m most interested in the implications of this work for astrobiology in general. Let me quote from the paper on these laboratory analogues of Titan’s haze:
These molecules are for example good UV absorbers and thus modulate the radiative balance of the atmosphere (Brassé et al. 2015). This chemical structure would also influence the surface energy of the haze particles, controlling their wettability with liquid/solid hydrocarbons and nitriles: it would impact their propensity to trigger methane rains in the troposphere and/or to transiently float at the lake surfaces of Titan (Cordier & Carrasco 2019; Yu et al. 2020).
And keep this in mind for the overall context:
More generally this work showed the potential of AFM technique to reveal the chemical structure of complex organic material of interest for astrochemistry, opening new perspectives in the chemical analysis of rare and complex material such as organic matter contained in meteorites or in the frame of future sample return missions.
The paper is Schulz et al., “Imaging Titan’s Organic Haze at Atomic Scale,” Astrophysical Journal Letters Vol. 908, No. 1 (12 February 2021). Abstract / Full Text.



The AFM images are just incredible (see the paper). I had only seen metals before, so these images that clearly show the bond density between the carbon atoms are amazing to me.
I’m not sure how relevant these studies are to astrobiology. The Miller-Urey experiment and others produced amino acids, most of which are not polycyclic or have aromatic groups, and most contain oxygen, possibly due to the H2O or CO2 in the primordial atmosphere. We also know that AAs occur in space, so they are easy to form under a wide range of conditions.
If AAs formed first (and then proteins), this would support the metabolism first origin theory. At the time of the Miller-Urey experiment, the structure of DNA had not yet been elucidated, and its importance not fully understood. When the DNA->RNA->protein dogma was in play, the idea that DNA came first competed with the metabolism first hypothesis. This was strengthened when it was realized how much RNA is involved in life processes, even acting as enzymes. This led to the “RNA world” hypothesis which is actively pursued even though so far there has been limited progress in understanding how RNA could form abiotically.
I am intrigued by the idea that a tholin haze could have protected the Earth’s surface from macromolecule-destroying UV rays. However, water could have provided teh same protection. However, I do wonder about the idea that the haze particles might have been instrumental in allowing concentrated layers of macromolecules to form, obviating the need for the need to concentrate these molecules in drying volcanic muds to polymerize.
I’ve actually seen better AFM – this one actually visualized *hydrogen bonds*: https://io9.gizmodo.com/the-very-first-image-of-a-hydrogen-bond-1426759827
I agree about the “RNA world” invalidating Miller-Urey as a meaningful abiogenesis. However, I should first say that the idea was since reworked a bit: polymerized HCN could have directly produced peptides of a sort ( https://link.springer.com/chapter/10.1007%2F3-540-54752-5_195 ). Miller-Urey then *hydrolyzed* those back into amino acids! Polymerized HCN is also thought to be responsible for tholins. As for the RNA world, maybe there were other nucleotides, such as nicotinic acid/nicotinamide, flavins, alpha-ribazole 5′-phosphate, or xanthine and diaminopurine, which have been subsequently purged from a genome that serves almost exclusively as a recipe for proteins, but which once gave RNA all the acids, amides, and redox activity shown by amino acids and protein cofactors.
The idea that appeals to me most for abiogenesis begins with the mineral hydroxyapatite, which can act as a substrate for pentose (esp. ribose) formation: https://pubs.rsc.org/en/content/articlelanding/2017/OB/C7OB02051A#!divAbstract This puts us very close to phosophoribose, and from there any passing amine might react with the aldehyde position to become a sort of nucleotide. An intermediate in the initial formose reaction is three-carbon sugar. Phosophorylated glycerol is the skeleton of lipids, which have a surprising affinity for hydroxyapatite, forming struvites in old outhouses or plaques in arteries. For that matter, if five carbon sugars could react to become four to five carbon keto dicarboxylic acids that chelate calcium, you’d be on the verge of a working Krebs cycle. So the primordial life I imagine would have half a cell membrane, hydrophobic side outward, stretched across a little patch of exposed hydroxyapatite, supported by the disproportionation of oxygen in spontaneous reactions between sugars in the roofed space beneath them and on surrounding regions of the mineral. The creature(s) [counting seems futile] would “feed”/”respire” from free formaldehyde gas to make sugars, by adsorption of any free lipophilic compounds from the water, and by amines reacting with the anomeric carbons of the sugars. The resulting lawn of nucleotides would have a range of diffuse catalytic activities, and presumably, dreams of future grandeur.
I see what you mean. Sometimes I feel like I am so behind the times.
Kind of a digression but I designed a Petroleum Museum in Malaysia years ago. Back then, and maybe now, the science was firmly in the “oil is biogenic in origin due to burial and decay of organisms” camp.
But I’ve wondered, since Titan is covered in oceans of hydrocarbons that are presumably non-biological in origin, if Earth’s oil could in part be due to our ancient atmosphere’s Titan-like processes.
Can’t wait for the next mission!
What were/are your thoughts on Gold’s “Deep Hot Biosphere” theory to account for fossil fuel deposits? I know the drilling project in Sweden proved a bust, but has there been more evidence either way? The formation doesn’t have to be binary, but it could be a mixture of two processes. A coal deposit on Mars would be pretty spectacular – a bit like Haldane’s comment about evolution – that it would be disproved if they found “Fossil rabbits in the Precambrian.”
Alex,
He was a real outlier to my client in the mid 90s!
“Gold arrived at this conclusion from the humble observation that meteorites and other planetary bodies contain abundant and compositionally diverse hydrocarbons (e.g., the methane lakes of the Jovian moon Titan), begging the question as to why hydrocarbons in the deep subsurface of Earth need to have a different origin”
——–and———-
“Gold believed that biology is just a branch of thermodynamics, being supported by energy in disequilibria in chemical reactions that would otherwise equilibrate if they were not held up by kinetic or mechanistic traps”
From https://www.pnas.org/content/114/27/6895
When the Earth’s oil was produced, the atmosphere was more or less as it is now, so it is likely to be biological in origin.
Apparently, there is practically no oxygen (the element) in the atmosphere of Titan. No H2O, no CO2, and of course no O2. Thus, none of the amino acids or nucleotides could possibly be formed here, as they all contain oxygen. Therefore, it seems a little farfetched to draw parallels to early Earth or abiogenesis, in my view. Maybe it’s different when you involve the solid surface, which is made primarily from H2O, as I understand.
I was under the impression that reaction rates proceed according to temperature. Consequently I expect anything that might occur on Titan to do so at an absolutely glacial pace. Or not at all.
That’s a good objection, and tied into the lack of oxygen in gasses of Titan’s atmosphere. However, Titan has a classic icy-moon structure with tidal evidence of a water ocean under the ice: https://science.nasa.gov/science-news/science-at-nasa/2012/28jun_titanocean/ If there are any cryovolcanic vents leading up toward the surface (like https://www.space.com/10486-ice-volcano-saturn-moon-titan.html ), the organic chemistry of the atmosphere and the aqueous chemistry of the ocean should meet, possibly also at warm temperatures. I doubt anything very special happens when they do, but … you never know.
Just like with Europa and Enceladus, and probably many other similar worlds, just because it’s really cold on the surface doesn’t mean there aren’t very different – and perhaps life friendlier – conditions below. All that water is a sure sign right there that things are at odds.
In response to ““RNA world” hypothesis which is actively pursued even though so far there has been limited progress in understanding how RNA could form abiotically.”
Yesterday a paper came out describing experiments showing that modified tRNA molecules can self-assemble into a larger molecule which is capable of replicating information stored in its sequence. This was without the help of additional enzymes, and with a replication fidelity per basepair of ca 85%.
In modern cells, tRNA (or transfer RNA) are the molecules that translate genetic information encoded in RNA (which itself is copied from DNA) into proteins, in a process called translation. These experiments suggest that perhaps tRNA’s oldest function was not translation, but replication.
Paper is here:
https://elifesciences.org/articles/63431
Carl Sagan had the Miller-Urey experiment recreated on his original Cosmos series:
https://www.youtube.com/watch?v=_2xly_5Ei3U
What Is Life? Its Vast Diversity Defies Easy Definition.
Scientists have struggled to formulate a universal definition of life. Is it possible they don’t need one?
People often feel that they can intuitively recognize whether something is alive, but nature is filled with entities that flout easy categorization as life or non-life — and the challenge may intensify as other planets and moons open up to exploration.
In this excerpt from his new book, Life’s Edge: The Search for What It Means to Be Alive, published today, the science writer Carl Zimmer discusses scientists’ frustrated efforts to develop a universal definition of life.
https://www.quantamagazine.org/what-is-life-its-vast-diversity-defies-easy-definition-20210309/