Although we’ve been focusing lately on photosynthesis, radiolysis — the dissociation of molecules by ionizing radiation — can produce food and energy for life below the surface and in deep oceans. Our interest in surface conditions thus needs to be complemented by the investigation of what may lie within, as Alex Tolley explains in today’s essay. Indeed, biospheres in a planet’s crust could withstand even the destruction of all surface life. The possible range of microorganisms well beyond the conventional habitable zone defined by liquid water is wide, and while detecting it will be challenging, we may be able to investigate the possibilities in our own system with landers, looking to a day when interstellar probes are possible to explore exoplanet interiors.
by Alex Tolley

“There may be only one garden of Eden here for large life forms such as ourselves. But living beings small enough to populate tiny pore spaces may well exist within several – and perhaps many-other planetary bodies.”
– Thomas Gold, The Deep Hot Biosphere, 1999 [1]
Thomas Gold was probably wrong about subsurface microbes being the source of fossil fuels using fossil methane (CH4), but he was the first to suggest that the newly discovered microbes in the Earth’s crust might be common in other planetary bodies. This essay will explore whether the molecules and energy available from the radiolysis of water (H2O) might support similar biospheres in other worlds in space.
Follow the water, but don’t forget the energy
NASA’s mantra of “follow the water” is important when searching for life, because liquid water is required for carbon-based terrestrial life. Life can still exist if the water freezes, but it will be in a non-metabolizing state and dormant. [Even in frozen water, such as the snow on mountains, a speck of dark material can melt a tiny volume of water around it, allowing microbes to live in these microscopic habitats.]
But while liquid water is necessary, it is insufficient to support life. Inoculate microbes in a dark, sealed flask of distilled water and they will die or go dormant, unable to acquire the energy needed for metabolism. [This is why you can keep containers of distilled water for a long time, even if bacteria contaminate the contents before sealing.]
The rich surface biosphere on Earth is powered by the sun. Photosynthesis fixes the sun’s energy from carbon dioxide (CO2) and water. Before photosynthesis evolved energy was anaerobically harvested from molecules that could liberate energy when respired. Bacteria living in the ocean’s dark, hot smoker vents metabolise the molecules erupting from the mantle and in turn provide the food and energy for the complex life living near these vents.
By the mid-1990s it was accepted that microorganisms discovered kilometers down in the crust were active in the interstices between the mineral grains. Water percolating in these rocks was responsible for keeping these microorganisms actively metabolizing rather than being in a dormant state. But what were they using for food and energy where it was lightless? Carbon was available as CO2 and CH4. The archaea kingdom of anaerobic organisms include all the methanogens that can convert CO2 to CH4 extracting energy and the using the carbon for metabolism. These were the dominant forms of life on and in the early Earth, and possibly the source of the traces of seasonal CH4 detected on Mars.
However, there is another more energetic molecule, molecular hydrogen (H2) that can be used for metabolism. As an electron donor, it can be coupled with an electron acceptor to be part of an energy harvesting metabolism. If H2 is a metabolic energy source, what is its source in the crustal biosphere?
The standard explanation is that some forms of the serpentinization reactions can produce H2 as well as the better known production of CH4.
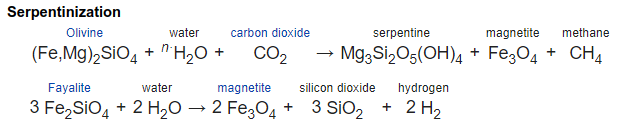
However there is another source of H2, created by radiolysis of H2O by decaying radioactive elements such as unstable isotopes of potassium (K), thorium (Th), and uranium (U).
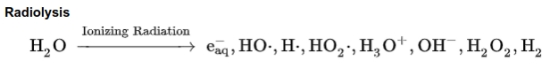
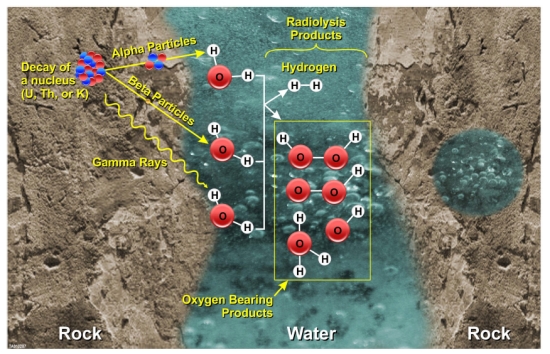
Figure 1. Radiolysis of water in the interstices of rock when water is present.
Besides creating H2, radiolysis also produces oxidants which in turn react with the rocks, most notably with sulfides, producing sulfates.
Li et al
“We have demonstrated that the S-MIF-bearing dissolved sulfate in the saline fracture waters at Kidd Creek originates from sulfides in the Archaean host rocks. The most likely mechanism for sulfate production in these anoxic fracture water systems is the indirect oxidation of sulfide minerals by oxidants from radiolytic decomposition of water” [3]
Radiolysis vs Serpentinization
Experiments on the radiolysis of water suggested that radiolysis was not an important source of H2 compared to serpentinization. Serpentinization occurs wherever high iron (Fe) igneous rocks from the mantle, water and heat interact. The ocean ridges between the plates are important zones where this takes place.
However, later experiments with oceanic sediments showed that radiolysis production of H2 was catalyzed by the minerals increasing production of H2 many fold. In the sediments on the ocean floor it was found that radiolysis was the main source of H2 as an energy source for microbes.
Sauvage et al: [9]
“Radiolytic H2 has been identified as the primary electron donor (food) for microorganisms in continental aquifers kilometers below Earth’s surface. […] all common marine sediment types catalyse radiolytic H2 production, amplifying yields by up to 27X relative to pure water. […] Comparison of radiolytic H2 consumption rates to organic oxidation rates suggests that water radiolysis is the principal source of biologically accessible energy for microbial communities in marine sediment older than a few million years.”
Moreover, radiolytic H2 is as dominant a source of food and energy as marine photosynthesis powered by the sun.
Sauvage et al: [10]
“[…] radiolytic H2 production in marine sediment locally produces as much electron donor (food) as photosynthetic carbon fixation in the ocean.”
In summary globally, radiolysis can provide both food and energy comparable to that of the marine photosynthetic organisms.
Methanogens & Sulfur-reducing bacteria
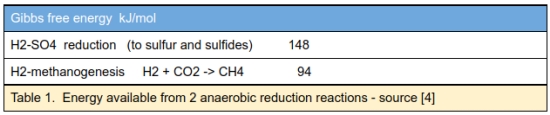
In anoxic environments where both archaeal methanogens and sulfur-reducing bacteria coexist, the biomass and types of the latter are greater than the former. One reason may be that the available energy from the reduction of sulphur from sulfates is greater than the reduction of carbon to CH4 from CO2.
CH4 can be created by the reduction of carbon dioxide.
![]()
Serpentinization is a geologic source. However, archaean methanogens are believed the dominant source of methane in the atmosphere on the early Earth reducing carbon dioxide anaerobically to methane [12]. It is the uncertainty of the source of the methane detected on Mars that intrigues astrobiologists.
Sulfate-reducing bacteria such as Desulfovibrio and Desulfobacter use the H2 to reduce sulfates created by the radiolysis oxidants on mineral sulfides to again reduce the sulfur to silfides.
![]()
As table 1 indicates, this is a more energetic reaction than methanogenesis and may account for the very many different bacteria utilizing H2 and sulphate as an energy source.The radiolytic oxidants also react with CH4 to form simple organic molecules such as formate and acetate which can be used as food sources by bacteria, further indicating the value of radiolysis in maintaining a subsurface habitat.
Habitability
As I have noted in previous posts, the search for life has typically been focused on surface-living, complex, aerobic life, as the low hanging fruit of detectability. This restricts the search to planets in the habitable zone (HZ). However, as unicellular life dominated Earth’s history and anaerobic respiration was dominant until the evolution of photosynthesis, and the Great Oxidation Event increased the partial pressure of oxygen in the atmosphere, such worlds may give rise to false negatives when analyzing the atmosphere by spectroscopy. Furthermore, as professor Tyrrell has suggested, surface life on Earth-like worlds may have a low probability of being sustained over 3 billion years due to events perturbing the surface temperature into runaway conditions.
Unlike the variable conditions on the surface, subject to wide ranges of conditions and vulnerable to cosmic and geologic disruption that saw 5 major extinctions on Earth, as well as an ongoing 6th extinction in the Anthropocene, conditions in the crust are far more stable, and less vulnerable to the disruptions on the surface. Such crustal biospheres once established may survive even after surface life has been extinguished, especially once the star’s luminosity renders the surface uninhabitable.
Such a biosphere may even allow for an evolutionary reset starting with microorganisms should surface conditions become uninhabitable for a temporary period and subsequently returning to habitability.
So we have evidence that life is in the subsurface crustal rocks and that radiolysis may be an important, if not the most important, source of food and energy for this life. But what about bodies elsewhere?
Other Celestial Bodies
1. Mars
Mars has all the same ingredients as Earth for subsurface microorganisms to live. At some depth below the surface the temperature should be sufficient to create liquid water [13]. While serpenitization can occur, especially to generate CH4, CO2 should be available for methanogens to respire and release CH4. Residual radioactive elements should be able to produce the needed H2 and SO4.for sulfur reducing bacteria. The race is on to determine whether there is an extant microbial biosphere on Mars. Looking for frozen microbes in ejecta from large meteor impacts that have penetrated to the needed depths might be the easiest approach for robotic vehicle discovery. If there are any near surface hot spots from residual volcanism or local concentrations of radioactive elements, these might also be good places to look. Whether earth, Venus, or Mars is the original world where abiogenesis occurred, panspermia between these worlds due to ejecta and microbes propelled by solar radiation, early Mars may have been a home for life.
Tarnas et al: [14]
“We have demonstrated that radiolysis alone produced sufficient quantities of reductants to have sustained a subsurface biosphere during the Noachian for hundreds of millions of years. Given sufficient oxidant availability, this habitat could have fostered chemolithotrophic microbial communities that would have imprinted organic, morphological, and isotopic biosignatures on their habitat’s host rock.”
2. Icy moons
Jupiter’s Europa and Saturn’s Enceladus are 2 icy moons that have subsurface oceans and tidally induced warming. Radiolysis of subsurface water in the core below the ocean sediments should provide the conditions necessary for a microbial biosphere. Analysis of the plumes by a flyby or orbiting probe is one method to search for life, although this is more likely to detect life in the oceans, rather than below the ocean-crust interface. This later will prove a much more difficult target.
3. Titan
Saturn’s moon Titan is believed to have a rocky core, overlaid with a liquid ocean, and topped with hydrocarbons with a dense nitrogen atmosphere. As with the icy moons, below the crust-ocean interface is a possible biosphere.
4. Ceres
Like the icy moons, Ceres has a rocky core overlaid with brines. Evidence of cryovolcanism suggests that these brines must be partially liquid. Unlike the icy moons, there is no tidal heating. This suggests that any biosphere in the core must be powered by radiolysis from any residual radioactive element decay. Catillo-Rogers recently suggested Ceres has the potential to host life as it has the radioactive elements for both heating and radiolysis. [5]
5. Eris
The Trans-Neptunian dwarf planet Eris has a density of 2.52 g/cm^3, indicating that it must be composed of rocky material and ices. If, like Pluto, there is evidence of cryovolcanism then it is possible that a core with radioactive elements and liquid water provides a habitat for a microbial biosphere. If so, then other dwarf planets extending out into the Kuiper belt could also have similar subsurface habitats.
6. Comets and Kuiper belt Objects
Holm focused on serpentinization for the production of CH4 and H2 on celestial bodies [7] He notes that radioactivity could also warm these bodies, making serpentinization possible. However, he did not consider radiolysis that might have been an important contribution to the production of energy rich molecules that could be used for metabolism.
Comets and their parent bodies, such as Transneptunian Objects (Kuiper Belt Objects—KBOs), accreted from a mixture of volatile ices, carbonaceous matter, and rocks in the coldest regions of the protosolar nebula. […] However, the rocky material contained in comets includes radioactive isotopes, whose decay can provide an important source of heat, possibly significantly altering the internal structure of these icy objects after their formation. There is a general agreement that short-lived radioactive isotopes like 26Al and 60Fe could have played a major role during the early evolution of both comets and their parent bodies, possibly leading to the melting of water ice and to the triggering of serpentinization and FTT reactions.
A more recent paper by Bouquet emphasized the importance of radiolysis in icy bodies which not only produced H2, but sulfates to support metabolism [2].
We found that radiolysis can produce H2 quantities equivalent to a few percent of what is estimated from serpentinization. Higher porosity, which is unlikely at the scale of a body’s entire core but possible just under the seafloor, can increase radiolytic production by almost an order of magnitude. The products of water radiolysis also include several oxidants, allowing for production of life-sustaining sulfates. Though previously unrecognized in this capacity, radiolysis in an ocean world’s outer core could be a fundamental agent in generating the chemical energy that could support life.
7. Rogue/Free Floating Planets
Rogue planets ejected from their systems would include bodies similar to those in the solar system. Given the prevalence of conditions needed for a subsurface biosphere, especially in bodies at the edge of our system, there seems every reason to believe that these rogue planets should also host subsurface conditions suitable for a microbial biosphere.
Habitable yes, but inhabited?
The above suggests that if radioactive elements can also heat the surrounding material so that water is kept liquid, then almost any celestial body with a rocky material and water could potentially be a microbial habitat in space, irrespective of whether it is in the HZ or not. As suggested earlier, planets in the HZ that have lost surface habitability could retain refugia for life in the crust.
For other non-Earth-like bodies which may have the conditions for a subsurface biosphere the question becomes whether they are living or sterile. Is abiogenesis possible on these worlds, or must they be inoculated by life from living worlds? We don’t yet know the answers to such questions, but it does suggest that astrobiologists take seriously the possibility that any body with a suitable subsurface environment could be inhabited and therefore instruments to detect such life should be included with exploratory probes. As we increase the exploration of our system, landers and rovers should include technologies to detect life, especially within the habitable zone below the surface.
Could we detect subsurface biospheres on exoplanets?
Detection of subsurface biospheres on exoplanets is going to be very difficult. Seager [6] produced a catalog of possible biosignature molecules, of which hydrogen sulphide (H2S) is primarily of biologic origin and therefore its presence is likely an unambiguous biosignature.
Although H2S is likely to have a very low concentration in the atmosphere it has a distinctive IR signal which could be detectable in principle,
As we can currently only analyze exoplanets spectroscopically, if it becomes possible to detect the very small amounts H2S in an otherwise unpromising atmosphere with possibly unsuitable surface conditions for life, then we should attempt to devise the technology to detect the presence of this gas as an unambiguous biosignature.
Based on the terrestrial history of life, it seems likely that on living exoplanets, extant life will be mostly unicellular, possibly even just prokaryotes. On Earth-like worlds geologic processes will ensure that such life will also inhabit a deep, crustal biosphere. Detecting such life will be very difficult, but perhaps not impossible with suitable technology. In the distant future, interstellar probes with landers should be able to detect such life as we explore our stellar neighborhood and catalog and map the forms of life we find.
References
Gold, T. (2021). The Deep Hot Biosphere: The Myth of Fossil Fuels (November 6, 1998) Hardcover. Springer.
Bouquet, A., Glein, C. R., Wyrick, D., & Waite, J. H. (2017). Alternative Energy: Production of H 2 by Radiolysis of Water in the Rocky Cores of Icy Bodies. The Astrophysical Journal, 840(1), L8. https://doi.org/10.3847/2041-8213/aa6d56
Li, L., Wing, B. A., Bui, T. H., McDermott, J. M., Slater, G. F., Wei, S., Lacrampe-Couloume, G., & Lollar, B. S. (2016). Sulfur mass-independent fractionation in subsurface fracture waters indicates a long-standing sulfur cycle in Precambrian rocks. Nature Communications, 7(1). https://doi.org/10.1038/ncomms13252
Lin, L. H., Wang, P. L., Rumble, D., Lippmann-Pipke, J., Boice, E., Pratt, L. M., Lollar, B. S., Brodie, E. L., Hazen, T. C., Andersen, G. L., DeSantis, T. Z., Moser, D. P., Kershaw, D., & Onstott, T. C. (2006). Long-Term Sustainability of a High-Energy, Low-Diversity Crustal Biome. Science, 314(5798), 479-482. https://doi.org/10.1126/science.1127376
Castillo-Rogez, J. C., Neveu, M., Scully, J. E., House, C. H., Quick, L. C., Bouquet, A., Miller, K., Bland, M., De Sanctis, M. C., Ermakov, A., Hendrix, A. R., Prettyman, T. H., Raymond, C. A., Russell, C. T., Sherwood, B. E., & Young, E. (2020). Ceres: Astrobiological Target and Possible Ocean World. Astrobiology, 20(2), 269-291. https://doi.org/10.1089/ast.2018.1999
Seager, S., Bains, W., & Petkowski, J. (2016). Toward a List of Molecules as Potential Biosignature Gases for the Search for Life on Exoplanets and Applications to Terrestrial Biochemistry. Astrobiology, 16(6), 465-485. https://doi.org/10.1089/ast.2015.1404
Holm, N., Oze, C., Mousis, O., Waite, J., & Guilbert-Lepoutre, A. (2015). Serpentinization and the Formation of H2 and CH4 on Celestial Bodies (Planets, Moons, Comets). Astrobiology, 15(7), 587-600. https://doi.org/10.1089/ast.2014.1188
Lollar, B. S., Onstott, T. C., Lacrampe-Couloume, G., & Ballentine, C. J. (2014). The contribution of the Precambrian continental lithosphere to global H2 production. Nature, 516(7531), 379-382. https://doi.org/10.1038/nature14017
Sauvage, J. F., Flinders, A., Spivack, A. J., Pockalny, R., Dunlea, A. G., Anderson, C. H., Smith, D. C., Murray, R. W., & D’Hondt, S. (2021). The contribution of water radiolysis to marine sedimentary life. Nature Communications, 12(1). https://doi.org/10.1038/s41467-021-21218-z
Sauvage, J. F., (2018) Sedimentary Catalysis of Radiolytic Hydrogen Production, Open Access Dissertations. Paper 704. https://digitalcommons.uri.edu/oa_diss/704
Cepelewicz, J, (2021, May 24). Radioactivity May Fuel Life Deep Underground and Inside Other Worlds, Quanta Magazine, Accessed online June 2021 https://www.quantamagazine.org/radioactivity-may-fuel-life-deep-underground-and-inside-other-worlds-20210524/
Tolley, A., Detecting Early Life on Exoplanets. February 23, 2018
https://www.centauri-dreams.org/2018/02/23/detecting-early-life-on-exoplanets/
Tolley, A., Is Most Life in the Universe Lithophilic? – AMT prior article JANUARY 11, 2019 https://www.centauri-dreams.org/2019/01/11/is-most-life-in-the-universe-lithophilic/
Tarnas, J., Mustard, J., Sherwood Lollar, B., Bramble, M., Cannon, K., Palumbo, A., & Plesa, A. C. (2018). Radiolytic H2 production on Noachian Mars: Implications for habitability and atmospheric warming. Earth and Planetary Science Letters, 502, 133-145. https://doi.org/10.1016/j.epsl.2018.09.001



Yes, this use of the term “habitable zone” to describe the Earth-analog zone around a star was out of date as soon as it was invented.
We won’t really know what the situation of life in the Galaxy is until we have capable interstellar probes, able where necessary to drill down under an exoplanet’s surface. It’s a bit disappointing that Project Icarus doesn’t yet seem to have published its final report, given that it was launched nearly 12 years ago (30 Sept. 2009).
Astrobiology’s fixation on complex, surface life is a chauvinism, dressed up as a claim that this is the only form of life our instruments have a shot at detecting. There are times when I imagine astrobiologists slashing their way with machete’s through exotic jungles like a Dan Dare strip.
If life is purely marine, then the detection of chlorophyll is possible, but the signal is weaker (even today our satellites are mostly used to detect harmful algal blooms) with possibly low atmospheric O2 levels prior to and during that world’s great oxidation event. Subsurface life, whether in the crustal rocks and sediments or in subsurface oceans is ignored as they are presumed undetectable, or at least that career-enhancing first detection of life will be made on an exoplanet with vegetation on the continents. IMO, given the slow pace and expense of solar system exploration, even if there is extraterrestrial life unless it is quickly discovered on Mars, the first detection of life elsewhere is most likely to be on an exoplanet. If a major search of the systems within a bubble of space of several light-years in radius turns up no biosignatures, that might be a blow to hopes that complex life and even oxygenic photosynthesis is ubiquitous and common. It would certainly reduce the probability of intelligent life as per the Drake equation that SETI programs are based on.
If most life is bacterial then we will need interstellar probes, but that would imply that without FTL, any discovery of life will be many decades, even centuries away.
And what did happen with project Icarus? Loss of interest after Breakthrough Starshot hogged the media?
I believe Icarus crashed after he flew to close to the sun… :-)
Speaking of deep dives…the inventor of the Jim suit has gone one better with Exosuit 2000 according to macleans.ca. -in fact, Nuytco uses once-classified submarine steel alloys only recently made available to industry. Good for Sea Dragon, Starship and Europa probes-but I would still fill them with liquid breathing-CRAY coolant bots for benthic exploration.
In round numbers, the amount of solar energy reaching the land and the oceans is 10,000 times the heat coming from radioactive delay from below. In terms of biomass or net metabolism, the deep biosphere will be sparsely populated. Even so, we’d love to find a living example one day.
So 2 issues will offset the apparent relative scale of biomass in the 2 biospheres.
1. Photosynthetic efficiency vs radiolysis efficiency to provide reduced carbon.
2. Differences in metabolic rates.
Of the two, I suspect [2] is most important. Metabolism, especially in cold sediments below the ocean is going to be very slow. We have some knowledge of this. In the crust, a lack of suitable molecules (H2 and H2O) probably ensures that metabolisms are just “ticking over”.
So the resulting biomass may be comparable to that in the oceans but the rate of metabolism is low. I would certainly not suggest at this stage that subsurface life is as energetically rich as the surface. Perhaps I should have made that clearer in the text.
From the perspective of finding life, which is better:
1. An extremely sparse distribution of bacteria that metabolize like those on the warm surface, or
2. A more numerous bacterial biota but with very slow metabolisms.
Hi Alex! You state that there is energy in the conversion of CO2 to CH4. I highly doubt that, since the opposite, i.e. the burning of CH4, releases a lot of energy. Instead, I submit that the most likely source of energy (and carbon!) for the earliest metabolism is the reaction of CO with water which can yield CO2 plus organic hydrocarbons. For example, 3CO + 2H2O —> CO2 + CH3-COOH yields acetic acid and, if I am not mistaken, some extra free energy. Acetic acid is your basic most primitive multicarbon compound that all biological molecules are derived from.
Interestingly, and in line with your radiolysis hypothesis, in the presence of CO2, CO and H2 are somewhat interchangeable, because CO + H2O CO2 + H2 with, I believe, an equilibrium at standard conditions.
While methanogenesis as a net energy source may seem counterintuitive, it is not. The Sabatier reaction to convert CO2 and H2 to CH4 is energetically favorable. This is the reaction that Zubrin proposed to create rocket fuel on Mars starting with a low-mass H2 supply.
So while CH4 + 2O2 -> CO2 + 2H2O produces net energy, this doesn’t invalidate the energy extraction by methanogens.
You are right, but it’s the H2 that is the important part. I may have misunderstood, but I thought you were saying that there was a path without the H2 (or CO) as the reductant.
That part wasn’t wrong – methanogens *do* extract energy by converting CO2 to CH4. But they need H2 to make this happen, and the way this was written might have given the wrong idea.
Oddly, it just came out that this reaction *can* happen even when oxygen is present, which seems like a terrible wasted opportunity, but here it is: https://www.pnas.org/content/118/27/e2019229118
Further to the CO hypothesis of early metabolism, note that a prime candidate for the oldest carbon fixation pathway is the Wood-Jungdahl cycle:
https://en.m.wikipedia.org/wiki/Wood–Ljungdahl_pathway
Note that the cycle begins with conversion of H2 to CO, so would work in the presence of CO and absence of H2 by simply skipping that step.
The questions raised in the post can only be answered by studying ‘pristine’ worlds, uncontaminated by human activities. We should not only continue to make sure that our space probes do not accidentally spread Earth life, but vigorously oppose any plans to ‘seed’ Earth life to other worlds. Whatever the case may be with natural panspermia from Earth, we should do our best not to add to it.
Assuming that perfect sterility is impossible, what should we do – study worlds with landers or abandon such studies entirely? And if the study really requires humans in the loop on those worlds, should we ban human presence too?
My view, just as we do science on earth, is that we do the best we can. We study subsurface bacteria on Earth by inspecting drilling cores and muds brought up in the course of drilling with human-managed rigs. Humans go down into mines to extract samples of water and rocks with as little contamination as possible. Given the size of the subsurface and how slowly organisms can migrate in that surface, it seems to me that the risks of contamination are relatively low.
If you are talking about directed panspermia and terraforming worlds, then it will depend on what “prime directive” is in force in that future. Unless there is an authoritarian government, my guess is that this will prove impossible to enforce, especially if a world looks like it will be suitable (and profitable?) to terraform. The Weyland-Yutani Corp would probably give the middle finger to the United Federation of Planets. How much adherence to planetary protection will the Chinese observe with their nascent space exploration?
Is your position that life should not be spread to worlds without life? Or, that worlds with life must be protected from introduction of other living organisms? There is quite a difference in those two positions.
My position is that truly sterile worlds should have life spread to them. I think that is a “gift” based on my bias as a living agent. I also adhere to Clarke’s view that mind is the most important thing that evolves (so far) and that by spreading life we are aiding that goal.
I also believe that we should refrain from contaminating already living worlds – the “Prime Directive”. But this requires an authoritarian unified system to achieve this, a solution I do not particularly like as it can lead to other behavior that is regulated. I also doubt it is possible, any more than we can stop METI by individuals, or wilful contamination, such as Zubrin’s view of Mars colonization. Our history (and speculative fiction of futures) is replete with willful disregard of life and others. Topical: gold mining in the Amazon, and mining the seabed for metal nodules in the Pacific given the go-ahead by Nauru despite a current halt by the UN, and of course the exploitation of other solar system bodies that required bending (breaking?) the UN treaties on space resources. So the Prime Directive is an idea I tend to favor but expect to be breached.
I don’t have a problem with landers (properly sterilized), or human exploration with precautions against contamination, although I expect the latter is decades or centuries in the future, even within the solar system (except for the Moon and maybe Mars and near-Earth asteroids).
We should not assume that any world is lifeless until it has been thoroughly explored, and even then I’d be hesitant about seeding it with Earth life. The possibilities of accident seem to me to exceed any possible benefit. A ban on seeding (even if practically unenforceable) would be best for the foreseeable future.
What accidents are you thinking about? Terraforming worlds could be done in different ways, although I think approaching it like new volcanic islands to colonize the continents is one way to go that would work fairly quickly for colonists. The slow way is to recapitulate evolution so that you start with bacteria and then add complex animals incrementally as near complex terrestrial ecosystem analogs as possible.
One question wrt terraforming planets with sub surface life only.
On Earth how much interaction is there between the subsurface ecology and the surface ecology?
As we didn’t have much of a clue that the subsurface achea existed until recently, I suspect that the answer is not much. Is this correct?
As to Mars, if it proves to be dead, surface & below, no restrictions on importing life, including introducing subsurface life from Earth. It would be a fascinating experiment.
If there is subsurface life, and interactions are limited, as I suspect, then terraforming could be restricted to introducing Earth surface (including soil bacteria that are part of the surface ecology). Mars & Earth life could then co-evolve together, another fascinating experiment.
There seems to be a general bias that Earth life would drive out existing Mars life. That might not be the case.
AFAIK that is likely true. Subsurface life represents a separate biosphere with very little interaction.
It would be hard to introduce subsurface life. Best left to see if it develops after terraforming.
Mars surface geology is nor active as it is on Earth. IDK if windborne dust and sand slowly accumulate as sediment or whether after its active geological period in the past Mars is preserving its surface apart from impacts.
If we terraform Mars so that we create flowing water and lakes or seas, then sediments could build up and earth life could be buried in those sediments and become relatively shallow subsurface life. Maybe water percolation might extend the downward range of microorganisms.
As regards co-evolving, I suspect that this would depend on the biological similarity between earth and Mars life. If they are the same, then horizontal gene transfer between bacteria is possible. Even if they are different, symbiotic coexistence could evolve, where only the exchange of metabolically compatible molecules could occur. If either life encapsulates the bacteria of the other, creating a potential future organelle, that would indeed be fascinating.
In KSR’s “Ultima”, in the distant future Earth and Proxima life eventually co-evolve, after billions of years.
IDK if that bias exists. I do think that it is a possibility that the science community wants to avoid in the event that native Martians exist. Humans do seem to have a propensity to destroy things, even accidentally. I think Joni Mitchell’s song, Big Yellow Taxi captures this idea very well.
Two possibilities that may be related;
1. Radiotrophic fungi.
https://en.wikipedia.org/wiki/Radiotrophic_fungus
2. Panspermia.
Carbon and high water content asteroids like Ryugu and Bennu. We may not have long to wait to see if Ryugu samples have some hitchhikers along for the ride and Bennu is throwing off rocks to impregnate the solar system.
Could be an interesting possibility with Radiolytic H2…
Large impactors also create their environment and energy sources for life after the impact, kind of like a potted plant, in the residual heat and chemical and water interactions in the crater that is formed. Now that is a perfect example of panspermia. Now if we find this kind of life off earth then a whole new field of astrobiology would be born.
One area that would be interesting to look at is ice and water in lava tubes on our moon and Mars – Transient Lunar Phenomena (TLP)??? Methane on Mars???
The wider point about radiolysis (and your fungus example) is that we have tended to assume that radiation is BAD FOR BIOLOGY. Hence the idea of galactic HZs away from the core, and the problem of flare stars and life. It is possible that some forms of life might prefer higher much radiation levels, as long as the balance between metabolism and molecular stability is positive for metabolism. The best example we have on Earth is the bacterium, Deinococcous radiodurans. Cosmic rays may be far too damaging, but higher intensity radiation from radioactive decay, protons, and electrons from a planet’s flare star, etc., may be a positive for life, not the negative we assume based on most of terrestrial life.
I would have thought impactors would have limited heat impact over time. Using your analogy, it is like potting a plant but neglecting to water it. I think impact ejecta is likely to be the main source of lithopanspermia in a system. What might be interesting is if the impacts between asteroids and other small objects act as a transmission vector in a similar way disease are spread between organisms in proximity.
Cosmic radiation is far more damaging on larger organisms than smaller ones, cosmic rays hits hard but the resultant spray of energy and particles does not damage the rest of the small organism because it’s not there to hit. Provided the organism can repair the damaged DNA/ RNA then it goes about its business once again.
This is what I’m talking about, the cross section of the crater explains it perfectly.
Ancient Life Signs Under Dinosaur-Killing Chicxulub Crater.
https://earthsky.org/space/dinosaur-killing-chicxulub-impact-crater-hydrothermal-microbial/
Another article on the possiability of even small impacts can create conditions for life.
Did meteorite impacts help create life on Earth and beyond?
Our understanding of the effects of impact events is continually evolving.
“A major aspect of our research has been documenting hydrothermal systems in impact craters around the world, to the point where we think it likely that most craters over a few km in diameter on Earth, and possibly Mars, would have generated hydrothermal systems.”
https://www.canadiangeographic.ca/article/did-meteorite-impacts-help-create-life-earth-and-beyond
The Role of Meteorite Impacts in the Origin of Life.
“The role that impact cratering plays in fracturing planetary crusts and its effects on deep subsurface habitats for life are also discussed.”
https://www.liebertpub.com/doi/10.1089/ast.2019.2203
The early earth would have a higher number and more energetic radioactive elements because one of the main forces that cause stellar gas clouds to collapse and form stars are supernovas. The supernovas release huge quantities of newly formed radioactive elements that are incorporated in the new stellar systems. The early solid earth would have plenty of radioactive energy to sustain and initiate deep earth life.
Impactors would fracture the surface deeply and create hydrothermal systems, so-called “lithophytic” habitats. The impacts also creates pumice like material that surface base life can hide in to protect themselves from UV radiation.
The section below is from “The Role of Meteorite Impacts in the Origin of Life.” with the link in the above comment.
4.3.?Deep subsurface habitats
In recent years, there has been a growing recognition of the importance of the deep subsurface biosphere on Earth (e.g., Gold, 1992; D’Hondt et al.,2002; Parkes et al.,2011; Lollar et al.,2014; Onstott et al.,2019). While previously thought to be either minor components of the terrestrial microbial biomass or simply dying transplanted surficial communities, the subsurface biosphere is now estimated to account for 5–15% of all biomass on Earth comprising 27–64 Gt of carbon and hosting over 90% of all bacteria and archaeal biomass on Earth (Bar-On et al.,2018; Magnabosco et al.,2018). Data from these studies derives from drill cores and from deep underground mines primarily in the shield areas of Canada, South Africa, and Scandinavia in rocks varying from 2 to 3 Ga. These regions represent the ancient highly metamorphosed cores of continents and, while important on Earth, would not have been formed and exhumed if it were not for plate tectonics, which, to our knowledge, is lacking on all other objects in the Solar System at the present day.
In the previous section we showed that fracturing, shock metamorphism, and melting during the thermobaric phase can create new surficial endolithic habitats. That meteorite impacts result in faulting and fracturing to kilometers’ depth is well established, both through field observations (e.g., Kenkmann et al.,2014) and gravity data (e.g., Pilkington and Grieve, 1992). Seismic studies of various craters on Earth demonstrate that the faults, particularly in the rims of complex impact craters, can penetrate several kilometers, with the maximum fault depth scaling approximately with the size of the crater (e.g., Pilkington and Grieve, 1992). However, it was not until the Gravity Recovery and Interior Laboratory (GRAIL) mission returned new gravity maps of the Moon that it was realized how important impact fracturing and faulting is on a planetary scale. GRAIL data revealed that the average porosity of the lunar crust is ?12% and that this high porosity extends to depths of at least 10–25?km and possibly down to the mantle (Wieczorek et al.,2013). Soderblom et al. (2015) further demonstrated that impact-generated fracturing is likely responsible for this high porosity.
Impact cratering thus provides a mechanism to fracture and fault planetary crusts down to several kilometers’ depth. Perhaps as important is that these faults are connected to the surface, providing a pathway to connect the previously described hydrothermal, lithophytic, and crater lake habitats with the deep subsurface. Indeed, the vast majority of microorganisms in the subsurface are substrate-attached outnumbering pelagic or free-living cells by 1–3 orders of magnitude (McMahon and Parnell, 2014). The significance of substrate attachment underscores the importance of rock surface area and physicochemical characteristics highlighting the role that both impact-induced fracturing and shock metamorphism play in creating subsurface habitats.
Is there any evidence that impact events have influenced the deep subsurface biosphere on Earth? Investigations on the microbiology of a 1.76?km drill core obtained from the Chesapeake Bay impact structure in the United States (Gohn et al.,2008), with robust contamination control (Cockell et al.,2009a, 2012b; Gronstal et al.,2009; Sanford et al.,2009), showed a logarithmic downward decline in cell abundance consistent with the general trend of decreasing biomass with increasing depth. However, cell enumerations within the impact breccia revealed much more abundant biomass levels than would have been predicted based on the general trend indicated by the post-impact lithologies. When compared to previously studied subsurface environments, these communities are found to be consistent with a microbiota influenced by the diverse and mixed lithologies present in the impact melt-bearing breccias. Coupled with the low hydraulic conductivity, the data suggest the microbial community remains influenced by the impact ?35 million years ago (Cockell et al.,2009a). While the discovery of relatively high biomass in impactite units at depth is intriguing, detailed metagenomic and biogeochemical studies are required to model the energy regimes and metabolic potential of subsurface impact environments. These data show that although impacts will sterilize the immediate area during the event itself, the fracturing caused by impact can yield enhanced habitat for microorganisms over the long term.
So if there is subsurface life on Mars, perhaps from its early wet period, then the subsequent cratering may well have created more habitat to compensate for the lack of geologic activity. A very interesting hypothesis, and one that future Martian astrobiologists may want to investigate.
Large impactors create a sea of lava, not the ideal conditions for germs.
Thank you for a great review.
Adaptability may extend habitability to the edge of survivability, without differentiation between surviving and thriving on that edge; “habitable zones” may be constricted and restricted to individual fissures in rocks, but microbes may be unaware of this.
Abiogenesis as we presume it to be, is a demanding process, exacting much from the milieu. The scale required may be met by miniaturization in the rock crevice; multiple episodes of abiogenesis may lead to multiple instances of fitness ab initio for the variant environments – perhaps implying their lesser fitness to earth’s environment.
It’s a long way from there to peeping out of the crevice
—ooO-(•/\•)-Ooo— but it’s a beginning.
As has been pointed out, an ubiquity of microbes with an absence of spacefaring civilizations would suggest the Great Filter is somewhere between the two. If non-spacefaring civilizations are many, The Great Filter may yet be ahead of us.
Alex:
Thank you for posting this superb, interesting piece!
Does the fact that all life on Earth has the same underlying biochemistry and, so far, despite numerous attempts, no life has arisen from scratch in the laboratory suggest to you that abiogenesis is extremely rare in the Universe? Does the fact that there have not been any independent origins of life discovered on Earth suggest that abiogenesis is a fluke because what would have precluded the emergence of several different biochemistries during Earth’s 3-4 billion years of habitability?
A solution to the Fermi paradox could answer your question. There are no aliens because if there were, they would have spread through the galaxy and left no room for us to evolve. Our existence is used as evidence against the existence of aliens.
Prebiology would not be able to compete with biology for resources. They would be food. Abiogenesis would transform the ecosystem into somewhere abiogenesis couldn’t happen.
While disappointing in one regard – we are alone with no one else to communicate with, it does offer us a blank slate to put our stamp on the galaxy. One possibility is that there are predatory civilizations out there, but they have not reached us yet. Pest control has either not found us, or they are on the way. In many respects it might be a lot safer if we are alone, or at least the first interstellar space-faring civilization should we go in that direction.
Those are possibilities. My response to Spaceman doesn’t reflect my approach to the Fermi paradox. I was using it as an analog that hopefully answered their question.
I don’t think all “break through events” such as abiogenesis lead to total domination of all niches. The evolution of a cellular power plant like chloroplasts was a break through event that did not prevent a similar break through event, mitochrondia, from occurring. That being said, abiogenesis would very likely preclude future abiogenesis. Regardless of the paradigm; rna first, metabolism first, or a synthesis of both, pre-biology looks too fragile.
The first or last paradigm says it borrows logic from nature. But when we look at nature we see first, then second layered on top, then third filling a niche neither first or second even comprehend. Why couldn’t the first make the galaxy more hospitable? Is first or last logic or aesthetic positioning?
The possibility of a 2nd abiogenesis is the theoretical basis for Paul Davies’ group to search for a “shadow biosphere”. Of course, if they truly find one, then the question is where that abiogenesis originated – on Earth, or elsewhere.
Aerobic life did not completely drive anaerobic life to extinction. Refugia in anoxic habitats allow anaerobes to exist. Metabolisms retain the ability to respire anaerobically too.
The idea of a galactic civilization or species driving out all others is possibly a reflection of how humans have done this throughout history. This may be a consequence of our primate-derived tribalism. Add in the common trope of alien invasions (c.f. the latest sci-fi movie “The Tomorrow War”) and one can see why our fears are reinforced – aliens are doing what we have done to others. But having said this, we know that species can eliminate others as our introduction of non-native species into new places attests. Generally, evolution by natural selection mostly happens within a population, not between them. The most competition is between individuals of the same species competing for the same resources, leaving little room to find a different niche already likely occupied by another species. Cultural evolution allows humans to create artificially separated populations, although so far those populations still compete for the same resources.
Thank you for the essay and response.
The probability that a break-out event precludes subsequent events depends on the how the requirements for the event map to the environment. If the event requires a broad supply chain of components and leads to a disruption of the supply of those components then we shouldn’t expect subsequent events. I lean towards a thermodynamic description for life, a paradigm that from my understanding requires a broad supply chain for components. Perhaps abiogenesis depends on a more narrow supply chain of components. Life transforms the environment. For multiple abiogenesis events to occur, the supply chain for abiogenesis would have to be narrow or abiogenesis events would have to be closely spaced in time, event B occurs after event A but before event A can transform the environment.
I try to be optimistic about ETI but there may not be many niches where people can survive Deep Time. The galaxy may look quiet only because we can’t see those niches.
We cannot answer the question of the frequency of abiogenesis from what we know so far. A “few” lab experiments may not capture the essential conditions needed as we don’t know what they are.
The lack of shadow biosphere organisms is expected as the current biology almost certainly outcompeted any others and drove them to extinction. There may have been several abiogenesis events and we would not know of them.
I think we really have to wait until we have data from other worlds – frustrating as this is, unless we get lucky with our experiments. A 2nd genesis in the solar system would be very indicative. Exoplanets will not help, because, as Astronist noted above, we need interstellar probes to analyze any exoplanet life and that won’t happen for a long time and the results will take even longer to receive.
“As has been pointed out, an ubiquity of microbes with an absence of spacefaring civilizations would suggest the Great Filter is somewhere between the two. If non-spacefaring civilizations are many, The Great Filter may yet be ahead of us.”
The assumption is always made that once consciousness arises, it should lead to a space proficient and space consuming civ. It is equally (nay, possibly much more) likely that advanced civs will choose to go virtual and/or enter a more interesting universe. It could be that a virtual (and “globally” accessible) universe exists, much like the web, that once you reach a sufficient level of tech you can access and connect with other civs. And, if the “Tinder” equivalent match up works out, then maybe you could have MIRL encounter…
Maybe the filter is that if you don’t reach that level of tech to access the universal web, and instead try for the stars, you will be filtered out! Life and domination of the greater physical universe may just be too incompatible.
I want to go to the stars, see suns set, but ultimately, is that just romanticism and childishness? The more I think about it the more I’m left with, “what is there to learn from 1(/1000000) light years away, that you couldn’t learn from where you are”. You don’t need to be at the edge of a blackhole, white dwarf or a red giant physically to know that such things exist and how they work. Indeed being too close to them will likely curtail your ability to understand them for much longer…. Once you have a handle on the broader “algorithm” the current one, i.e. the universe, becomes irrelevant.
You must be an armchair traveler by instinct. ;) At least some humans have a desire to travel and experience places. Some even engage in dangerous pursuits to be up close with those dangers. Even the best VR is a shadow of reality (so far). I would like to stand on the Moon and put my [space suited] fingers into the dust, perhaps even visit the locations where some favorite SciFi story was set to compare reality with fiction.
As I never needed the adrenaline rush of danger, for some things I would happily use VR – standing atop Everest, diving into the ocean trenches, walking into an active volcano. These are best served by robotic avatars that I control from my armchair.
Given the distances, I think experiencing the rings of Saturn is best done by passive VR, as is visiting locations on Mars, or Titan. Without FTL, I doubt anyone is going to watch a black hole from a vantage point near its event horizon unless a planet nearby has been colonized.
A very well supported and scholarly paper by Alex Tolley. The problem with it is not anything is written about the chemical and environmental processes needed to make chemical composition of the DNA which makes the Goldilocks zone crucial to biotic life. CH4 and H2 in the spectra only indicate some of the waste products of life and an environment that has or once had liquid water but not necessarily life, i.e., there can be there can be serpentinization without life. More is needed like lightning and ultraviolet light. Don’t forget the Miller-Urey experiment which was used by Carl Sagan in Cosmos. Consequently, I am still biased in favor of life originating near the surface where sunlight has helped create the necessary organic compounds. Life then migrated to more challenging environments like the thermophiles in the ocean.
The Miller-Urey experiment only dealt with the creation of amino acids. It has nothing to do with DNA, RNA, lipids, etc. There are competing theories for abiogenesis, including oceanic vents, where clearly UV and lightning don’t exist. So abiogenesis may occur in darwin’s “warm pond”, or it may be elsewhere. The latter is possibly favored as methanogens are all Archaean extremophiles which might (or might not) be relevant to the question.
If abiogenesis just requires a warm mafic rock, water, and energy to split that water (unlikely to be sufficient conditions, but we don’t know), then abiogenesis might be common. But even if that is not the case, the same conditions could offer a habitat for life spreading from elsewhere. No abiogenesis required, just the probability of some form of panspermia being the mechanism.
In summary, I don’t think that a world has to be in the HZ to act as a habitat for microbial life, any more than Earth has to be the only habitat for a space-faring human (or ET) civilization. If it was, then why even consider SETI strategies that include looking for megastructures in space? If life was restricted to the HZ, then Mars is just inside the outer edge, but none of the other planets are in the HZ, so there would presumably be no point in even trying to detect life elsewhere in the solar system. Astrobiologists clearly beg to differ on that point and very expensive robotic probes are being designed to look for life as part of their instrument payload. While I personally am not optimistic, if we don’t even look, we are guaranteed to find nothing.
The backbone of the DNA contains carbon, nitrogen and phosphate which comes from the phosphorus cycle. https://www.sciencelearn.org.nz/resources/961-the-phosphorus-cycle
Both N and P are found in rocks so the rocks might be a good local source of these elements, perhaps liberated from the minerals in solution as nitrates and phosphates, before being metabolized to the states needed. Hydrogen ions after donating electrons are suitable acids to break down many minerals.
“The backbone of the DNA contains carbon,…” indeed. Could a fresh batch be conjured up in some mircobrewery?
Endosymbiosis has played an important role in the evolution of complex life. Many of relationships are between cells with wildly different metabolisms. Overall complexity may be linked to how many diverse metabolic pathways a planet can sustain. Planets with limited potential may host more refined, delicate versions of their metabolisms. Planets like Earth would host simpler, more robust versions of each unique metabolism as well as more powerful and complex combinations. Biomass could be equivalent.
The chemical and molecular formula for the DNA: Molecular Formula: C15H31N3O13P2. The molecular formula for the four base pairs or amino acids, adenine, cytosine, guanine, thymine all are made of the elements carbon, hydrogen and nitrogen so the other two missing elements in the DNA are only carbon and phosphate which are their backbone so the Urey Miller still does apply.
The radiolysis of water by UV light can occur in water early in Earth’s history. The habitable zone is important because cell process can’t function at the bottom of the sea on one of Jupiter or Saturn’s moons because the pressure is too great so cells or the DNA could not have been made there. It is the environmental conditions that matter also so the certain physical conditions must be met like the need for liquid water at normal vapor pressure of one atmosphere, the only place where there is the chemical mixing of all these elements in water that cells could form which is why the Goldilocks zone is important. Thirty three feet under the sea is equal to one Earth atmosphere of 1000 millibars. 66 feet would equal two atmospheres. The radiolysis of water occurs at the surface or near surface.
I have to reject the idea of panspermia because it would take too long to seed the galaxy as most comets and asteroids at solar system escape velocity of a solar system are too slow. Furthermore panspermia is just a red herring to direct the attention away from the hard problem of how life began since there would have to be an origin planet where life first evolved because it did not evolve on an inhospitable, inert environment in space on cosmic dust, meteors, asteroids, comets and planetesimals, and there would still need to be a lot of origin planets in order for panspermia to be efficient and not take too long. One might as well consider the anthropic principle and life evolving under the idea of necessity in a very fine tuned, habitable environment, abiogenesis and the Gaia hypothesis.
That assertion may not be true. This paper indicates that there were pressure tolerant fermentation bacteria that survived rapid pressurization up to 400 MPa, which roughly translates to a depth of 40 km on Earth, and deeper in lower gravity wells. It seems possible to me that a slower pressurization would select for the pressure-tolerant cells which would become the dominant population. We know that bacteria live at the bottom of the deep ocean, even in the trenches 10 km in depth. Subsurface organisms in the crust may add even more pressure. So I would not a priori rule out organisms at the bottom of a deep ocean based on pressure.
Regarding panspermia. I agree that it is a deflection if considering abiogenesis on Earth. It becomes analogous to “God created life”. However, panspermia may exist, either as a natural consequence of material ejection from systems, to directed panspermia by an ETI. That ‘Oumuamua and Borisov comets entered our system from another system tells me that exchange of material, and therefore possibly life, is theoretically possible, however low probability. With billions of years before our system was formed, there might be very many impacts on Earth from interstellar objects, of which only one carrying life and successfully seeding Earth is needed. Directed panspermia is a very different proposition with a much higher probability of success. We could be doing this in a few centuries should we have the knowledge and inclination to do so. It will even be inadvertent if humans colonize Mars this century.
This PNAS paper indicates that RNA nucleobases (A.C,G,U) can be made under a Miller-Urey type experiment using lasers as the energy source. Formation of nucleobases in a Miller–Urey reducing atmosphere
DNA, RNA and prions are capable of replication, as far as is presently known on Earth.
The cheela of The Dragon’s Egg and The Black Cloud represent hard science-based speculation on alternate methods for replication, sentience and intelligence. And sans speculation, we have laboratory demonstration with xeno nucleic acids of alternate modes of replication. Low energy resources do not necessarily preclude higher energy molecular structures; concentrating energy is routine in biological systems.
Prions replicate the way a falling domino replicates – by having someone or something to set them up first. To be technical, in most situations DNA doesn’t even replicate without some information first being copied back into RNA – for the Okazaki primers!
Thermodynamic laws are descriptions of probability distributed over time. Life does not break the law of entropy. Life is an improbable arrangement. It is hard not to see the effect of assuming entropy as a force has on the willingness to engage with a thermodynamic, metabolic rich description for Life.
Thermodynamics doesn’t add another force, it distributes the supply chain for rare arrangements of structure. The information dominant paradigm requires a perhaps miraculously long and narrow supply chain to deliver rare events. Perhaps a thermodynamic enriched paradigm makes abiogenesis more rare, requiring a more expansive supply chain , but Life more robust.
In the spirit of competitive speculation. Life looks like a weird state of matter. What happens when matter has enough math, information management resources, and starts calculating system description? Is the progression towards complexity easier to explain if we consider quorum sensing and fossils that may be an animal or improbably organized cells, as examples of the system changing scale? Those two examples would both require a way to leverage description of the system away from already established systems.
A thermodynamics enriched theory of Life isn’t Gaia.
Earth looks extremely habitable. It would be a source for panspermia and anything arriving is unlikely to survive contact. Great place to live but wouldn’t want to visit.
I am afraid I don’t understand what you are getting at here.
I think we all understand that life looks on its face as decreasing entropy in contravention of the 2nd law of thermodynamics, but in fact, using energy to do this. But beyond that?
So we have experiments that show precursor life molecules like amino acids can be formed by energy inputs changing suitable ingredients (Miller-Urey experiment and many others).
Stuart Kauffman has shown theoretically how you can get “order for free” by autocatalytic sets appearing when there is a sufficient diversity of substrates.
Darwinian natural selection is a powerful algorithm to keep increasing order and decreasing entropy, by pruning away comparative disorder.
But how this all translates into “thermodynamic supply chains”” a phrase I do not understand. What about “The information dominant paradigm requires a perhaps miraculously long and narrow supply chain to deliver rare events.”. Can you elaborate or provide a suitable reference?
Alex Tolley, I think you are over emphasizing the adaptability of the final, complete DNA after it already evolved. I am not the expert on biology, but it is the virus that is the most adaptable. One virus survived over a year on the Moon on the U.S. first moon lander, the Surveyor and the astronauts took a piece of it back to Earth and a virus was found in a compartment.. It was not in the sunlight though. Maybe bacteria can survive deep inside the crust, but that does not mean life evolved at those depths. There is not enough chemical mixing at that depth to get all the chemicals in the DNA. One has to think of a test tube or flash with water an then add all the right ingredients, the Miller, Urey experiment.
As far as Panspermia is concerned, I think you have accepted that idea uncritically. In my opinion, panspermia would be more like God created life without any science. I don’t think God is limited to the imagination and belief, but in the view of some contemporary, liberal scientists, God does not contradict science when one considers a priori, first principles like math and general relativity which control the death and birth of stars long before the birth of our solar system which were not only created by man. Life evolving on Earth from inanimate matter could still be considered to be divine considering the anthropic principle. We still don’t know how to replicate that process, but we have a good idea that is how it happened.
I have not said that abiogenesis occurred in the crust. We know that the bacteria found in the crust either can be identified with surface organisms or are very close matches. bacteria in teh ocean sediments below the ocean bottom are clearly identifiable as marine species. In both cases, the organisms migrated in some way from teh surface, either by sediment depostion, percolating water, or by subduction.
Regarding panspermia. Just suppose we found subsurface life on Mars and that it proved to have near identical biology to terrestrial life. Which is the more likely hypothesis?
1. There were 2 genesis events,and both resulted in the same biology.
2. There was a single genesis event and then a transfer of life to the 2nd workd.
I would favor hypothesis 2.
Panspermia within the solar system, particularly between the 3 rocky woprlds that may all have had surface water in their past – Venus, Earth, and Mars – needs to be considered if the data warrants it. We have Martian meterites so we know that theoretically, lithospanspermia is possible, however low a probability. We know bacteria can be found in the upper atmosphere and therefore it is also possible that there may be a transfer between worlds as they move into conjunction – Venus to Earth, Earth to Mars, Venus to Mars If we find any subsurface life on Mars and it is the same as on Earth, then panspermia by any of these 2 methods is a possibility, even likely.
Interstellar panspermia would appear to have a very low probability. However, with the recent revival of organisms many millions of years old, we cannot rule out that a long, cold, interstellar trip via a comet or rocky ejecta makes such a transfer of life impossible. The probability might be vanishingly low, but it may not be zero. The discovery of two interstellar comets raises that probability. I have also raised the suggestion that we should test interstellar dust for life. But this is not the only means of transfer. Tom Gold long ago proposed that life might have come to Earth from the contamination by an ET landing in the distant past after the Earth formed. More recently, as interstellar flight of micro packages seems within our grasp this century, that directed panspermia to deliberately seed a suitable [sterile] world is a possibility. Trip times are now based on fractions of c. Panspermia therefore must be a possibility to consider should we find life on other worlds that is very similar to terrestrial life.
Bottom line, I don’t consider panspermia as a likely event, but rather as a hypothesis that must be considered if the biology leads us in that direction.
Good work, Alex!
I wrote THE MARTIAN RACE 22 years ago to draw attention to the chemistries you treat. I included CH4 plus that hydrogen sulphide (H2S) is primarily of biologic origin here, so when life retreated from the early Mars surface (from atmosphere loss) it colonized the larger pores and passages available in Martian volcano times. That it persists can best be studied by autonomous rovers going into the many caves we know there, with nuclear power and microwave comm back to the surface. Then humans… as happens in that novel.
Although “The Martian Race” isn’t in my library (many moves seem to have resulted in books going astray) with other books by you, a quick read of the plot online triggered a recall of the discovery of the marsmat – perhaps a short story, or an excerpt?
I am also reminded of “Heart of the Comet” (which is in my library) that you co-authored with David Brin about life in Halley’s comet. Now that would be a major discovery if that ever happened! Whether acknowledged or not, the concept seems to have been used by several relatively recent SciFi movies.
Quote by Alex Tolley: “If we find any subsurface life on Mars and it is the same as on Earth, then panspermia by any of these 2 methods is a possibility, even likely.” If we find life on Mars and the DNA is exactly the same life on Earth, it also would prove that the DNA is the same everywhere in the universe based on the organizing principles of science which prove that the physical universe is ordered and controlled by general principles that allow us to make accurate predictions given the same physical environment and chemical processes and has nothing to do with migration, but only the high probability of instinctual necessity and evolution to produce the same forms everywhere.
The problem with interplanetary panspermia is it is a lot easier for us or the Earth to get a Martian meteor, or Moon meteor, than for Mars and the Moon to get an Earth meteor since Earth’s escape velocity is much higher than Mars and the Moon where a giant impact will easily cause debris to reach escape velocity. In my opinion panspermia is more myth than scientific fact.
When I say that if any life is found on Mars and it is very similar to terrestrial life, it is far more that than just the structure of DNA. It is about the genetic code (which is set largely by chance), a commonality between genes coding for key proteins which are unlikely to be the same by chance, and so on. If a phylogenetic tree shows that Mars life can be connected to the terrestrial life, then it is strong evidence that Mars and Earth life share a common ancestor. That would indicate some transference of life from one planet to the other, or that they share a common source from elsewhere. It would not suggest that the two forms of life evolved independently starting with separate abiogenesis events. That is getting perilously close to accepting creationism ofrintelligent design.
I recall Alex Tolley telling you I thought that the configuration of the DNA four base pairs formed through necessity and you replied in a comment that it was optimal. Optimal would be mutually exclusive from chance. My reasons are that what is random and chance is considered meaningless in physical science and statistics. I will admit that I am biased in favor of the anthropic principle because all of the physical contingencies necessary for life to survive long term on Earth, like a Moon for a fast rotation and magnetic field, and maybe even plate tectonics. The more we remove these contingencies, the less the probability for life . I could be wrong about this, and in the next five years we should be able to know with the spectra of nearby exoplanets. We shall see.
I thought I may have said that there was a paper saying the genetic code was optimal based on the existing codons and the 20 amino acids. Whether this was correct, or whether there was a range of codes close to optimum IDK. But even so, it assumes that the DNA structure, the bases used to pair up, the tRNA, and the amino acids are the same. Recently I read that there is a possibility that DNA once used only 2 bases rather than 4. Crick called the genetic code frozen by accident (ie by chance). We really need evidence of other life to find out.
If DNA structure, amino acids, and teh genetic code are universally the same all started from a different genesis, I would be very surprised and would like to see some sort of experiment to show why this should be so. Core metabolic biology would have to follow, again, some genes coding for core proteins would have to be very similar to terrestrial genes as well, all by convergent evolution. This all seems very unlikely, but it isn’t impossible.
You may wish to look at some of the papers in this Google search regarding genetic code optimality. You may need to think about what the authors are doing and the constraints they assume.
Google search on genetic code optimization
Going by what you have said Alley Tolley, the 2 base pairs are not optimal so they did not make it which is ordinary evolution. The final product is 4 base pairs which is most adaptable so considering an exactly similar environment on exoplanets, it is logical to assume a DNA with four base would be the same. It is an illusion that we have many different outward forms so like must be always changing and adapting which is true, but the inner form remains the same, i.e., life is controlled by necessity. Now I will admit that is an a priori assumption of mine, but one has to prove that the 4 base pairs are the result of necessity. I don’t know how to do that because I don’t have enough knowledge of biology, but only that necessity always picks what is the most optimal for survival according to Darwin. What isn’t optimal becomes extinct. One has to look at the final product since what does not make any sense of is superfluous always gets screened out.
Oldest fossils of methane-cycling microbes expand frontiers of habitability on early Earth
EUROPLANET/UNIVERSITY OF BOLOGNA PRESS RELEASE
A team of international researchers, led by the University of Bologna, has discovered the fossilised remains of methane-cycling microbes that lived in a hydrothermal system beneath the sea floor 3.42 billion years ago.
https://www.europlanet-society.org/oldest-fossils-of-methane-cycling-microbes-expand-frontiers-of-habitability-on-early-earth/
City-sized asteroids smacked ancient Earth 10 times more often than thought.
“The true impact flux could have been up to a factor of 10 times higher than previously thought in the period between 3.5 and 2.5 billion years ago.”
https://www.space.com/ancient-earth-hit-by-city-size-asteroids-often
Dinosaur-killing asteroid generated TSUNAMI nearly one mile high.
https://www.msn.com/en-us/weather/topstories/dinosaur-killing-asteroid-generated-tsunami-nearly-one-mile-high/ar-AAM9l25?ocid=U452DHP&li=BBnbfcL