Dave Moore is a Centauri Dreams regular who has long pursued an interest in the observation and exploration of deep space. He was born and raised in New Zealand, spent time in Australia, and now runs a small business in Klamath Falls, Oregon. He counts Arthur C. Clarke as a childhood hero, and science fiction as an impetus for his acquiring a degree in biology and chemistry. Dave has kept up an active interest in SETI (see If Loud Aliens Explain Human Earliness, Quiet Aliens Are Also Rare) as well as the exoplanet hunt, and today examines an unusual class of planets that is just now emerging as an active field of study.
by Dave Moore
Let me draw your attention to a paper with interesting implications for exoplanet habitability. The paper is “Potential long-term habitable conditions on planets with primordial H–He atmospheres,” by Marit Mol Lous, Ravit Helled and Christoph Mordasini. Published in Nature Astronomy, this paper is a follow-on to Madhusudhan et al’s paper on Hycean worlds. Paul’s article Hycean Worlds: A New Candidate for Biosignatures caught my imagination and led to this further look.
Both papers cover Super-Earths, planets larger than 120% of Earth’s radius, but smaller than the Sub-Neptunes, which are generally considered to start at twice Earth’s radius. Super-Earths occur around 40% of M-dwarf stars examined and are projected to constitute 30% of all planets, making them the most common type in the galaxy. Hycean planets are a postulated subgroup of Super-Earths that have a particular geology and chemistry; that is, they have a water layer above a rocky core below a hydrogen–helium primordial atmosphere.
We’ll be hearing a lot more about these worlds in the future. They are similar enough to Earth to be regarded as a good target for biomarkers, but being larger than Earth, they are easier to detect via stellar Doppler shift or stellar transit, and their deep atmospheres make obtaining their spectra easier than with terrestrial worlds. The James Webb telescope is marginal for this purpose, but getting detailed atmospheric spectra is well within the range of the next generation of giant, ground-based telescopes: the 39-meter Extremely Large Telescope and the 24.5-meter Giant Magellan Telescope, both of which are under construction and set to start collecting data by the end of the decade (the status of the Thirty Meter Telescope is still problematic).
Earth quickly lost its primordial hydrogen-helium atmosphere, but once a planet’s mass reaches 150% of Earth’s, this process slows considerably and planets more massive than that can retain their primordial atmosphere for gigayears. Hydrogen, being a simple molecule, does not have a lot of absorption lines in the infrared, but under pressure, the pressure-broadening of these lines makes it a passable greenhouse gas.
If the atmosphere is of the correct depth, this will allow surface water to persist over a much wider range of insolation than with Earth-like planets. With enough atmosphere, the insulating effect is sufficient to maintain temperate conditions over geological lengths of time from the planet’s internal heat flow alone, meaning these planets, with a sufficiently dense atmosphere, can have temperate surface conditions even if they have been ejected from planetary systems and wander the depths of space.
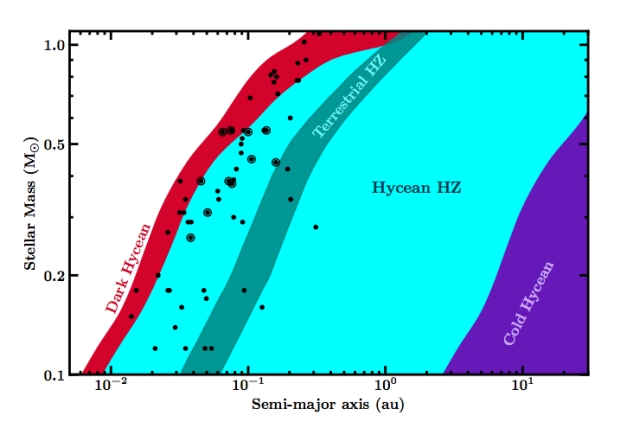
Figure 1: This is a chart from Madhusudhan et al’s paper showing the range where Hycean planets maintain surface temperatures suitable for liquid water, compared with the habitable zone for terrestrial planets as derived by Kopparapu et al. ‘Cold Hycean’ refers to planets where stellar insolation plays a negligible part in heating the surface. Keep in mind, that Lous et al regard the inner part of this zone as unviable due to atmospheric loss.
Madhusudhan et al’s models were a series of static snapshots under a variety of conditions. Lous et al’s paper builds on this by modeling the surface conditions of these planets over time. The authors take a star of solar luminosity with a solar evolutionary track and, using 1.5, 3 and 8 Earth mass planets, model the surface temperature over time at various distances and hydrogen overpressures, also calculating in the heat flow from radiogenic decay.
Typically, a planet will start off too hot. Its steam atmosphere will condense, leaving the planet with oceans; and after some period, the surface temperature will fall below freezing. The chart below shows the length of time a planet has a surface temperature that allows liquid water. (Note that, because of higher surface pressures, water in these scenarios has a boiling point well over 100°C, so the oceans may be considered inhospitable to life for parts of their range.)
Planets with small envelope masses have liquid water conditions relatively early on, while planets with more massive envelopes reach liquid water conditions later in their evolution. Out to 10 au, stellar insolation is the dominant factor in determining the surface temperature, but further out than that, the heat of radiogenic decay takes over. The authors use log M(atm)/log M(Earth) on their Y axis, which I didn’t find very helpful. To convert this to an approximate surface pressure in bars, make the following conversions: 10-6 = 1 bar, 10-5 = 10 bar, 10-4 = 100 bar and so on.
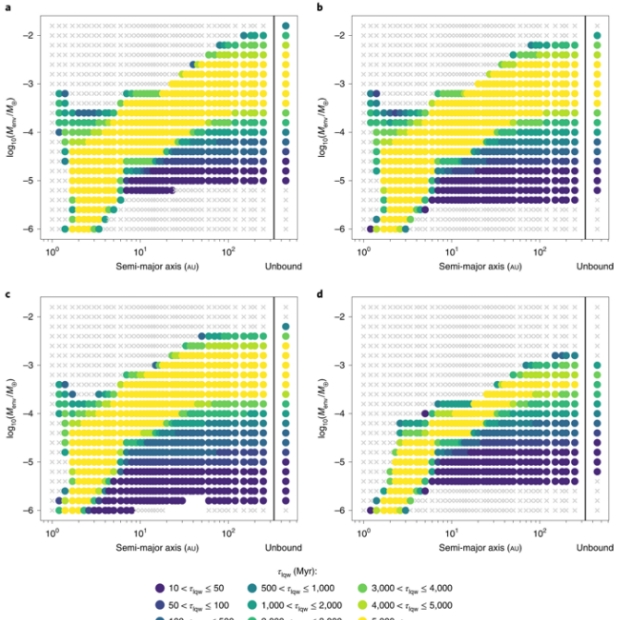
Figure 2: Charts a-c are for core masses of 1.5 (a), 3 (b) and 8 M? (c). The duration of the total evolution is 8 Gyr. The color of a grid point indicates how long there were continuous surface pressures and temperatures allowing liquid water, ?lqw. These range from 10 Myr (purple) to over 5 Gyr (yellow). Gray crosses correspond to cases with no liquid water conditions lasting longer than 10 Myr. Atmospheric loss is not considered in these simulations. d is the results for planets with a core mass of 3 M?, but including the constraint that the surface temperature must remain between 270 and 400 K. Every panel contains an ‘unbound’ case where the distance is set to 106 AU and solar insolation has become negligible.
The authors then ran their model adjusted for hydrodynamic escape (Jeans escape is negligible). This loss of atmosphere mainly affects the less massive, closer in planets with thinner atmospheres.
To quote:
The results when the hydrodynamic escape model is included are shown in Fig. 3. In this case, we find that there are no long-term liquid water conditions possible on planets with a primordial atmosphere within 2au. Madhusudhan et al. found that for planets around Sun-like stars, liquid water conditions are allowed at a distance of ~1 au. We find that the pressures required for liquid water conditions between 1 and 2au are too low to be resistant against atmospheric escape, assuming that the planet does not migrate at a late evolutionary stage.
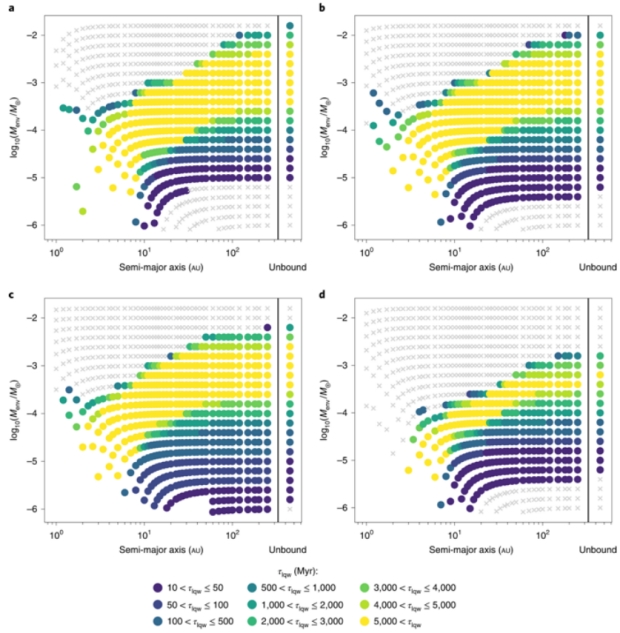
Figure 3: Charts a-c are for core masses of 1.5 (a), 3 (b) and 8 M? (c). d is the results for planets with a core mass of 3 M?, but including the constraint that the surface temperature must remain between 270 and 400 K. Note: escape inhibits liquid water conditions by removing the atmosphere for close-in planets with low initial envelope masses. Lower core masses are more affected.
The authors also note that their simulations indicate that, unlike terrestrial planets which require climatic negative feedback loops to retain temperate conditions, Hycean worlds are naturally stable over very long periods of time.
The authors then go on to discuss the possibility of life, pointing out that the surface pressures required are frequently in the 100 to 1000 bar range, which is the level of the deep ocean and with similar light levels, so photosynthesis is out. This is a problem searching for biomarkers because photosynthesis produces chemical disequilibria, which are considered a sign of biological activity, whereas chemotrophs, the sort of life forms you would expect to find, make their living by destroying chemical disequilibria.
The authors hope to do a similar analysis with red dwarf stars as these are the stars where Super-Earths occur most frequently. Also, they are the stars where the contrast between stellar and planetary luminosity gives the best signal.
Thoughts and Speculations
The exotic nature of these planets lead me to examine their properties, so here are some points I came up with that you may want to consider:
i) The Fulton Gap—also called the small planet mass-radius valley. Small planets around stars have a distinctly bimodal distribution with peaks at 1.3 Earth radii and 2.4 Earth radii with a minimum at 1.8 Earth radii. Density measurements align with this distribution. Super-Earth densities peak, on average, at 1.4 Earth radii with a steady fall off above that. Planets smaller than about 1.5 Earth radii are thought to contain a solid core with shallow atmospheres, whereas planets above 1.8 Earth radii are thought to have deep atmospheres of volatiles and a composition like an Ice-Giant (i.e. they are Sub-Neptunes.)
Taking Lous et al’s planets, a 3 Earth mass planet would have an approximate radius of 1.3 Earth radii. An 8 Earth mass planet would have an approximate radius of 1.8 Earth radii (assuming similar densities to Earth.) This would point towards the 8 Earth mass planets having an atmosphere too deep to make a Hycean world. The atmosphere would probably transition into a supercritical fluid.
ii) I compared the liquid water atmospheric pressures from our solar system’s giant planets with the expectations of the paper. I had trouble finding good figures, as the pressure temperature charts peter out at water ice cloud level, but here are the approximate figures for the giant planets compared with the range on the 270°K-400°K graph that Lous et al produced:
Jupiter: 7-11 bar / 8-30 bar
Saturn: 10-20 bar / 25-100 bar
Neptune: 50+ Bar (50 bar is the level at which ice clouds form) / 200-500 bar
Our giant planets appear to be on the shallow side of the paper’s expectations. This could be attributed to our giant planets having greater internal heat flow than the Super-Earths modeled, but that would make the deviation greatest for Jupiter and least for Neptune. The deviation, however, appears to increase in the other direction.
The authors of the paper note that their models did not take into consideration the greenhouse effect of other gasses such as ammonia and methane likely to be found in Hycean planets’ atmospheres, which would add to the greenhouse effect and therefore give a shallower pressure profile for a given temperature. And from looking at our giant planets, this would appear to be the case.
This could mean that an unbound world would maintain a liquid ocean under something like 100+ bars of atmosphere rather than the 1000 bars originally postulated.
iii) Next, I considered the chemistry of Hycean worlds. Using our solar system’s giant planets as a guide, we can expect considerable quantities of methane, ammonia, hydrogen sulfide and phosphine in the atmospheres of Hycean worlds. The methane would stay a gas, but ammonia, being highly hydrophilic, would dissolve into the ocean. If the planet’s nitrogen to water ratio is similar to Earth’s, this would result in an approximately 1% ammonia solution. A ratio like Jupiter’s would give a 13% solution. (Ammonia cleaning fluids are generally 1-3% in concentration.) A 1% solution would have a pH of about 12, but some of this alkalinity may be buffered by the hydrosulfide ion (HS–) from the hydrogen sulfide in solution.
It then occurred to me to look at freezing point depression curves of ammonia/water mixtures, and they are really gnarly. An ammonia/water ocean, if cooled below 0°C, will develop an ice cap, but as the water freezes out, this increases the ammonia concentration, causing a considerable depression in the freezing point. If the ocean reaches -60°C, something interesting starts to happen. The ice crystals forming in the ocean and floating up to the base of the ice cap start to sink, as the ocean fluid, now 25% ammonia, is less dense than ice. This will result in an overturn of the ocean and the ice cap. Further cooling will result in the continued precipitation of ice crystals until the ocean reaches a eutectic mixture of approximately 2 parts water to 1 part ammonia, which freezes at -91°C. (For comparison, pure ammonia freezes at -78°C.) Note: all figures are for 1 bar.
When discussing the possibility of liquid water on planets, we have to include the fact that water under sufficient pressure can be liquid up to its critical point of 374°C. The paper takes this into account; but what we see here is that, aside from showing that the range of insolation over which planets can have liquid water is larger than we thought, the range that water can be liquid is also larger than we assumed.
While some passing thought has been given to the possibility of ammonia as a solvent for life forms, nobody appears to have considered water/ammonia mixtures.
iv) Turning from ammonia to methane, I began to wonder if these planets would have a brown haze like Titan. A little bit of research showed that the brown haze of Titan is mainly made of tholins, which are formed by the UV photolysis of methane and nitrogen. Tholins are highly insoluble in hydrocarbons, which is why Titan’s lakes are relatively pure mixtures of hydrocarbons. However, tholins are highly soluble in polar solvents like water. So a Hycean planet with a water cycle would rain out tholins that formed in the upper atmosphere, but if the surface was frozen like Titan’s, they would stay in the atmosphere, forming a brown haze.
This points to the possibility that there are significant differences in the composition of a Hycean planet’s atmosphere depending on whether its surface is frozen or oceanic. and this may be detectable by spectroscopy.
I’m looking forward to finding out more about these planets. In some ways, I feel that in respect to exosolar planets, we are now in a position similar to that of our own solar system in the early 60s – eagerly awaiting the first details to come in.
References
Marit Mol Lous, Ravit Helled and Christoph Mordasini, “Potential long-term habitable conditions on planets with primordial H–He atmospheres,” Nature Astronomy, 6: 819-827 (July 2022). Full text.
Nikku Madhusudhan, Anjali A. A. Piette, and Savvas Constantinou, “Habitability and Biosignatures of Hycean Worlds,” The Astrophysical Journal, (Aug. 2021). Preprint.
Fulton et al, “The California-Kepler Survey. III. A Gap in the Radius Distribution of Small Planets,” The Astronomical Journal, 154 (3) 2017. Abstract.
Christopher P. McKay, ”Elemental composition, solubility, and optical properties of Titan’s organic haze,” Planetary Space Science, 8: 741-747 (1996). Abstract.



The combination of a hydrogen-helium atmosphere for the warmth to maintain water-ammonia as a liquid does seem like an interesting possibility for life, but why not look to Saturn first? Of course, in Saturn there is the problem of trace elements – does some traces of phosphine and germane etc. make for an interesting enough chemistry, or do we have to suppose that non-volatile compounds are brought up in storms at supercritical pressure? (or when storytelling, perhaps by a deeper ecosystem)
The idea of a smaller “Hycean” planet alone in deep space, relying on a very thick atmosphere to hold in its geologic warmth, seems more problematic to me. There is less internal energy than on Saturn and the energy *gradient* would be very low. In an environment where the hot and cold reservoirs are nearly the same temperature, under an insulating atmosphere without any holes (contrasted with Callisto tiger stripes) for interesting juxtapositions, nor sunlight shining through, there is little opportunity for thermodynamics to make anything happen. At least on Saturn there is a tremendous amount of energy in the turbulence of the atmosphere, which might not be the case for a sunless world.
Barring litigious native inhabitants, Saturn remains the planet of choice in our system for habitation by plain-vanilla humans. With Earthlike gravity and a vast hydrogen-helium atmosphere for fuel, nearly all we have to do is fire up our Mr. Fusions, pave over the primary atmosphere with some of the carbon, and breathe the waste nitrogen and oxygen we release above it. That’s 84,000 Earths worth of prime real estate. :)
Overall, many interesting thoughts in this article!
Very interesting, Dave! I remember previously reading about the possibility of rogue terrestrial planets with temperate conditions; it’s good to see the state of the theory move forward. Of course, anything that increases the possible environments for complex life is significant.
Even though the dense atmosphere and/or the unbound rogue nature of these planets have dark surfaces, that may not preclude photosynthesis. There has been some work that shows that infrared light from hot ocean vents can power photosynthesis. Obviously nowhere near as energetic as terrestrial sunlight, but possibly as productive as hydrogen/methane for chemotrophs.
I don’t know how frequently these worlds are, but it seems that they must be a fabulous resource for water to maintain chemical/nuclear propulsion and life support for biologicals like us. Maybe in the far future, such worlds may be mapped for worldships to visit and replenish their volatiles losses.
If a desert world and such a hycean world shared a system and both were in their respective HZs, then conceivably the water could be used to change the biosphere of the dry, desert world.
Lastly, you mention that large terrestrial telescopes will be able to detect potential biosignatures of these worlds. Those biosignatures will have to be limited to visible light spectra and exclude the useful IR spectra.
The 39 meter ELT is been build at 3000 meters in the Atacama desert. It is capable of detecting near IR. I wasn’t able to get its exact specs, but VLT build nearby has a wavelength range of 300nm-20 micron.
Thinking about the possible events that can knock a planet’s environmental stability such as our Earth has experienced over just the last 0.5 Gy, I wonder if such hycean worlds might be much more stable. A denser atmosphere to reduce impacts, lack of inflammable carbon on landmasses, no landmass configuration changes to change the climate, and in the case of these worlds that are unbound or far from their star, no real impact of GHG on the surface temperature?
While we can’t yet know if life has appeared on such worlds, if they are sterile we could seed them with terrestrial life – existing or engineered. Such worlds may become the galactic factories to produce vast quantities of biologicals for health and food.
Quote by Alex Toley: “andmasses, no landmass configuration changes to change the climate, and in the case of these worlds that are unbound or far from their star, no real impact of GHG on the surface temperature?” Absolutely. Super Earth’s would have frozen solid oceans in interplanetary space and even and oceans world’s atmosphere would freeze because there is not enough internal heat. A billion miles away the star would certainly freeze a super Earth and the surface of the ocean world.
The atmosphere of the super Earth would be completely frozen on it’s surface in interplanetary space in only distant starlight.
I thought the argument in the OP was that a super-Earth with a H2/He atmosphere would stay warm from internal heat. This would maintain the atmosphere as an insulating blanket. Are you saying that is false?
If so, how large would the planet have to be – a Brown Dwarf?
Interesting paper. We have to consider the planetology principle of jeans escape with Titan which kept is original atmosphere from the proto planetary gas cloud. The reason being is Titan’s distance from the Sun keeps the temperature cold enough, almost down to cryogenic temperatures. It would quickly loose it’s hydrocarbon atmosphere CH4 and most of its atmosphere including N2 if it was moved to the into our life belt because it is a smaller body than Mars with a lower escape velocity which is included in jeans escape. Five bars and over of any gas is a greenhouse gas do it’s density or thermal infrared radiation cross section. I don’t see NH3 freezing in polar caps on super Earths unless it is early in its history since those trace gases including hydrogen will evaporate and be mostly lost as it’s star become brighter over it’s history.
Very thought provoking article, but an area that may be interesting is the mixing of the material at the ocean to thermal geologic activity boundary. We see mixing in the trenches and mid oceanic ridges and it may be at a much higher rate and depth on Hycean Worlds then here on earth. The possibility of layering and high concentrations of certain inorganic chemicals and metals could make for unusual life forms. The interaction on these worlds would be different then the gas giants since no ices and metallic hydrogen would be between the dense core and the oceans and atmospheres. The heating and mixing from below could make a cosmic cesspool of organic and inorganic plus biometals to create a huge inventory of chemicals out of disequilibrium. Mixing for billions of years these could even show up in the spectral signatures in the planets atmosphere…
Metal based lifeforms, hmm.
“Various metals, together with oxygen, can form very complex and thermally stable structures rivaling those of organic compounds; the heteropoly acids are one such family. Some metal oxides are also similar to carbon in their ability to form both nanotube structures and diamond-like crystals (such as cubic zirconia). Titanium, aluminium, magnesium, and iron are all more abundant in the Earth’s crust than carbon. Metal-oxide-based life could therefore be a possibility under certain conditions, including those (such as high temperatures) at which carbon-based life would be unlikely. The Cronin group at Glasgow University reported self-assembly of tungsten polyoxometalates into cell-like spheres. By modifying their metal oxide content, the spheres can acquire holes that act as porous membrane, selectively allowing chemicals in and out of the sphere according to size.”
http://arcana.wikidot.com/metal-based-life
There is a potential habitable environment type for such lifeforms: molten salt oceans on hot evaporated mini-neptune cores.
Metals are indeed able to form very complex and diverse chains and other structures together with oxygen, maybe even more so than carbon-based compounds. Metals have higher coordination number (6 for most 3d-metals and 8 for heavier analogs vs. 4 for carbon) and much more diverse coordination geometries. At 300 K, metal-oxide structures are rather inert, just like our own biochemistry would be in liquid methane at the surface of Titan, but they are readily dissolved in molten salts and brought to full activation by elevated temperatures and dissolution.
There is much greater range of both possible solvents and building blocks than for carbon-based life. Molten-salt ocean could incorporate all combinations of salt cations (Na+, K+, Mg2+, Ca2+ to some extent) and anions (Cl-, Br-, SO4\2-, CO3\2-), and there is a broad range of full miscibility in liquid phase. Insoluble phase could include the metal-oxide chains themselves, polyoxometallate-class-structures (Mo, W, Nb, Ta and many other metals), silicate and phosphate-based fragments (cell membranes?), and many other structures which all come into play at red-hot temperatures.
Abiogenesis on such worlds would be rare because much wider range means much greater percentage of unsuitable combinations of conditions, solvents and dissolved building blocks. But a successful molten-salt-based biosphere could possibly develop much smaller and more efficient analogs of proteins, because of the same much greater numbers and versatilities of basic building blocks.
The hypothetical civilization development would be paradoxical. Energy fluxes orders of magnitude larger than in water HZ would greatly accelerate life, growth and evolution. Imagine an Earth’s year-long lifecycle of a metal-oxide forest, photosynthetizing at 100x Earth’s insolation. But the great heat needed by such life is readily radiated away in space, and technology does not work well at their room temperature of 800 – 1300 K. These people would be much more restricted to their planets and “natural ways”.
PS What is the best temperature range for technology? Maybe liquid-methane range, where superconductors don’t need chilling and the number of stable organic molecules increases by orders of magnitude? Most technology of liquid methane dwellers would burst into flames on Earth’s surface just like ours would in the molten salt, but there’s much more backward compatibility. A smartphone from MOx City Store could likely be reanimated after journey to Earth (or Titan) and back.
Technology may be best at liquid methane temps, but the biology to get there would be thermodynamically so slow…
What metals or materials for technology hardware be best at liquid CH4 temps as metals become brittle at these temps?
MOx life is an interesting idea, reminiscent of Clarke short story about a civilization living in our planet’s mantle. But realistically, do MOx compounds have the needed diversity to provide a biology based on molecular shape and energy transfer? Do you have any references for this speculative concept that answers the questions about their suitability for life?
MOx was almost pure speculation to me before the reference to the Cronin group work about polyoxometallates. I noticed the diversity of metal-oxide structures while studying chemistry, and thought that, given the right conditions, they could at least rival carbon compounds. I searched references and discussed this with enthusiasts from time to time, but nothing sparked. I haven’t found any definitely astrobiological references in the past, at least not striking my mind as “that’s it”.
In later 2010s I realized that molten salt oceans could be quite common – there are many hot mini-neptunes and their evaporated cores, with just right temperatures, – and that molten salts could turn out to be much better solvent for MOx than water. NaCl at 800 degrees is possibly too aggressive and hot, but eutectic mixtures at somewhat lower temps seem better. (what is liquidus temperature for sea salt? No refs at first glance but I guess around 700 oC) Carbon-carbon chains are disrupted at 500-600 K, and 300 K in water gives just right balance of reactivity and stability. This corresponds to, maybe, 1500 K for degradation of metal-oxygen chains and 600-1000 K for “balance temperature”. For MOx, it’s difficult to estimate, because there is much more variety and much less data, and my chemistry years are quite in the past :) But water, except maybe near-critical phase, is definitely too cold.
I can imagine difficulties with molten-salt oceans, though. Salt composition on mini-neptunes would be no doubt different from our ocean. Salt content is defined by percentage in the crust, not by volume of water. Global equivalent layer of salt in Earth oceans is around 30 meters compared to 2 km of water. On mini-neptunes, maybe couple hundreds of meters could be leached because of more vigorous mixing, but is this enough for “salt hydrosphere”? Molten salts dissolve stuff too readily, the oceans would be nowhere near our clear blue water – more likely thick mud full of dissolved compounds. Or not? There are white carbonatite lavas on Earth, and Baltis Vallis channel on Venus where possibly molten-salt lava flowed thousands of kilometers without dissolving into it’s shores. Evaporated cores are tidally locked except around hot stars, eccentric orbits, wide separations with greenhouse-sustained heat.
OTOH, eccentric hot earths with molten salt oceans would have day-night cycles like Mercury, and vigorous volcanism to bring nutrients to the surface. Thick outgassed atmosphere could shield MOx life from freezing each night and expand “molten salt HZ” outwards quite like on Hycean worlds. Indeed, without reflective clouds, our Venus would be at it’s outer edge with surface temperature closer to 600 degrees – it’s just too “salt-dry”. (where are salt flats from it’s oceans? It should be many meters thick if there were any, at least somewhere on the surface)
Re. life and tech at liquid-methane temps: some metals and alloys become brittle and their performance degrades to some degree, but this is far from becoming unusable. The more significant problem is with metal availability on the surfaces of icy bodies with liquid methane. If the body is fully differentiated, with active surface and thick liquid layer beneath it, the sole metal source would be meteorites and space dust. Maybe trace salts in cryovolcanic lavas. Iron and nickel would be as precious as gold and other metals even more so. OTOH, gravity and V_escape on Titan is so low that orbit can be reached on rockets made fully from plastic, as well as small undifferentiated bodies in the same system where rocks just lie on the surface :)
An afterthought about temperature ranges. Life needs temperature range of chemical activation – below is definitely too cold. Liquid methane temp is around 0.15 of carbon-carbon chain degradation temperatures – if there is any life, it would need truly extreme adaptations. Very special biochemistry that would decompose in milliseconds at our room temperature. Machinery, in general, does not need chemical activation, and because of this, high-temperature tech is qualitatively more compatible with low temperatures than vice-versa. The general lower bound is zero instead of “half of material degradation temperature”, not counting uneven thermal expansion cracking, elecrolyte freezing and the like. Steel is worked at 800 degrees but works well at room temperature and only slightly worse – close to absolute zero. The same for most metals, silicon, etc.
Thanks Dave a great post
This is one of my favorite subjects so you have given me lots of papers to look up and read.
“Earth quickly lost its primordial hydrogen-helium atmosphere, but once a planet’s mass reaches 150% of Earth’s, ”
That’s interesting I thought it was higher to hold on to an envelope like 3-5 Earth Masses? I wounder how massive a planet has to be to hold onto its Hydrogen and helium?
Thanks Edwin
Edwin, I took the mass measurement that from the paper. The paper had a series of charts/graphs showing Hydrogen mass loss under various scenarios. The big variable for Hydrogen mass loss was distance from the primary. At 3 Earth mass planet with a 100 bar of Hydrogen loses its Hydrogen in 500 million years at 2 au. At 6 au, there’s a 20% loss over 10 billion years. Mass loss appears to be approximately linear, so 100 bar atmosphere will last 10 times that one a 10 bar atmosphere. With respect to mass, a 1.5 Earth mass planet loses its Hydrogen at approximately twice the rate of an 8 Earth mass planet.
How big could birds or some flying species be in a 100 bar Hydrogen atmosphere?
Flying whales? Perhaps not so much about size, but of the needed flight surfaces for lift and propulsion. Crudely, wings might be a small fraction of the size – more like fins and flippers rather than wings as we have on birds, bats, and insects. OTOH, if the atmosphere was H/He, that implies an anaerobic metabolism which is less energetic suggesting the animals would be sluggish, soaring after brief takeoff.
Hot hydrogen blimps with black surface like hot air balloons.
The idea of habitable zone hydrogen atmosphere planets has been around for a bit now. Sexy name though -Hycean. Care has to be taken in that they are only theoretical constructs as yet – not real planets sat out their in the cosmos. Sara Seager and her team at MIT have been pushing this idea since the late noughties – with a good introductory article in the Astrophysical journal, Nov 2010. More recently CD regular Ramses Ramirez has touched on the subject too via volcanically produced hydrogen atmospheres – as opposed to the primordial hydrogen left over version described above. For those interested in the background and evolution of the idea – especially of finding bio signatures .
Where this article adds is in more detailed atmospheric modelling . When telescopes with the ability to spectroscopically classify potentially habitable atmospheres finally come on line they need to know what to look for. What does a ( not too ) hydrogen rich thin envelope habitable atmosphere spectrum look like in practice ? It’s no good just pointing your telescope and creating a tell tale spectroscopic readout with stand out features . You might as well just read tea leaves at the bottom of a cup. Creating detailed ( 3D) atmospheric models of all potential planetary atmospheres – from none at all through thin envelope CO2 all the way up to deep hot Jovian , is going to be essential. Then any recovered exoplanetary atmospheric spectra can be compared and classified.
This is going to be the particularly relevant to the first potential habitable atmospheres coming out of the 39.3 E-ELT from circa 2027 onwards. It is the only one of the three ELTs that will have a first generation instrument , the mid IR ( M band) imaging ( with coronagraph) spectrograph, METIS and associated extreme Adaptive optics potentially capable of habitable exoplanetary science . Hopefully. METIS will be able to pluck nearby exoplanetary spectra from the ( substantial) confounding background spectra of the accompanying star as well as the Earth’s own atmosphere. Probably – via the use of the high definition imaging method proposed by CD contributor Ignas Snellen in 2013. The coronagraphic spectrographic imager on the Nancy Roman space telescope is unlikely to have the sensitivity and resolution to do this and neither of the other two ELTs have a first generation instrument capable of sophisticated analysis required for habitable exoplanet science. We’ll be lucky to see a large dedicated exoplanetary space telescope this side of 2050 I’m afraid.
It’s critical meantime that the astrophysics and geophysics community get modelling on their super computers so we can recognise what METIS finds. ( the ESA ELT website describes METIS in detail)
Hi Dave & Paul
This discussion reminds strongly of Hal Clement’s Kainui (“Great Ocean”) from ‘Noise’ (2003). Radius of 1.15 Earth and yet a surface gravity of 1/3 gee, due to a global ocean 2,900 kilometres deep. Hot at the bottom it’s continually full of lethal shockwaves from seismic activity where ocean meets mantle. Where Kainui differs is from having an N2/CO2 atmosphere. But a H2/CH4 atmosphere further from a star is entirely feasible.